Effect of preganglionar denervation on the expression of adrenergic, muscarinic, androgen, and prolactin receptors at the major pelvic ganglion of long-term sexual behaving male rats
- Inicio
- Comité Editorial
- Lineamientos
- Carta de Cesión de Derechos
- Información Legal
- Acerca de la Revista
- Bases de Datos
- Contacto
- ISSN 2007-3054
- Centro de Investigaciones Cerebrales
Universidad Veracruzana
Alejandro Mateos-Moreno1, Viridiana Sánchez-Zavaleta1, Gonzalo E. Aranda-Abreu2, Deissy Herrera-Covarrubias2, Fausto Rojas-Durán2, Jorge Manzo2, Jorge Suárez-Medellín2, Ma. Rebeca Toledo2, Ma Elena Hernández-Aguilar2*
1Doctorado en Investigaciones Cerebrales, Universidad Veracruzana, Xalapa, Veracruz, México.
2Instituto de Investigaciones Cerebrales, Universidad Veracruzana, Xalapa, Veracruz, México.
Abstract
Resumen
Introduction
Materials and Methods
Results
Discussion
Conclusion
Conflict of interests
Acknowledgements
References
Mail
The major pelvic ganglion is an autonomic complex comprised of a mixture of noradrenergic and cholinergic fibers aimed to innervate most viscera at the pelvic area. To accomplish this, its neurons express receptors to androgen, norepinephrine and acetylcholine ligands. It is known that sexual behavior produces both, an important activity of neuron groups inside the ganglion, and a systemic increase of prolactin and testosterone. The link between both effects is observed following the axotomy of pelvic and hypogastric nerves. Thus, the aim to determine how these receptors are modified by preganglionic denervation of male rats. The expression of adrenergic, cholinergic, androgen, and prolactin receptors in the major pelvic ganglion was analyzed by Western Blots in sexually expert and denervated subjects. Results showed that sexual behavior does not promote changes in protein density, but denervation induces an increase in androgen and muscarinic receptor levels. It is suggested that the increased expression of these receptors could be a plastic compensatory mechanism following denervation maybe to respond to endocrine influences and maintain the function of the ganglion.
Keywords: Pelvic Nerve, Hypogastric Nerve, Autonomic Neurotransmission.
El ganglio pélvico mayor es un complejo autonómico compuesto por una mezcla de fibras noradrenérgicas y colinérgicas cuyo objetivo es inervar órganos de la visera pélvica. Para llevar a cabo esto, las neuronas expresan receptores a andrógenos, noradrenalina y acetilcolina. Se conoce que la conducta sexual produce una importante actividad neuronal dentro del ganglio, así como incremento sistémico de prolactina y testosterona. La relación entre ambos efectos se observa posterior a la axotomía de los nervios pélvico e hipogástrico. Es por ello por lo que el objetivo de este estudio es determinar cómo estos receptores son modificados por denervación preganglionar en ratas macho. La expresión de los receptores adrenérgicos, colinérgicos, andrógenos y prolactina en el ganglio pélvico mayor fue analizada mediante western Blot en sujetos sexualmente expertos y denervados. Los resultados mostraron que la conducta sexual no promueve cambios en la densidad de ninguno de los receptores analizados, pero la denervación indujo incremento en los niveles de los receptores a andrógenos y muscarínico. Lo que sugiere que el incremento en la expresión de estos receptores podría ser un mecanismo plástico compensatorio debido a la denervación y tal vez para responder a la influencia endocrina y el mantenimiento de la función del ganglio.
Palabras clave: Nervio pélvico, Nervio hipogástrico, Neurotransmisión autónoma.
The major pelvic ganglion (MPG) is an autonomic complex of the peripheral nervous system that receive preganglionic fibers from the pelvic (Pv) and hypogastric (Hg) nerves that supply the ganglion neurons responsible of regulating the functions of pelvic viscera, including the prostate, seminal vesicles, penis, rectum, testicle and bladder.1 The MPG is composed of approximately 14,000 noradrenergic and cholinergic ganglion neurons, accompanied also by small intensely fluorescent cells (SIF), satellite cells, Schwann cells and vascular endothelial cells.2 The ganglionic neurons and SIF cells express a number of receptors including adrenergic, cholinergic, androgen, adenosine, ATP, among others, that have an important role in ganglion function.1,3-5 Androgens promote cell growth through androgen receptors localized in neurons and SIF cells.6 It is known that alterations in the neurotransmission from Pv and Hg nerves produce changes in the prostate cytoarchitecture suggesting that this gland is under both hormonal and nervous control.2,7 MPG preganglionic denervation induces changes in the expression of transcriptional factors such as c-jun and transcription factor 3 (ATF-3), as well as increased expression of neuropeptide galanin (GAL), proteins associated with cell death and neuroprotection,5 ,8 hence all these molecular changes are responsible for the observed lesions in the prostate.
Moreover, neural activation has been observed following sexual behavior whereas in the MPG Fos-IR expression was positively correlated with the amount of both genital and noncontact stimulation.9 On the other hand, sexual behavior induces an increase in systemic levels of prolactin and testosterone and an increase of prolactin and androgens receptors at the prostate gland.10,11 Notwithstanding, there is no information about how receptors in the MPG are affected by preganglionic denervation in sexually behaving rats. Hence, the purpose of this work was to analyze the effect of long-time sexual behavior and denervation on androgen, prolactin, adrenergic, and cholinergic receptors.
2.1. Subjects
The experiments used sexually experienced Wistar male rats from Harlan, Mexico (250-300 g/bw), they were kept in Plexiglas cages (50 x 30 x 20 cm) in a temperature-controlled room (22oC) with free access to food (Harlan rodent chow) and water. The room was kept at a reversed 12:12 cycle (lights on at 20:00 h). Care of animals was approved by the ethics committee of the Instituto de Investigaciones Cerebrales of Universidad Veracruzana in accordance with the policies for the use of animals in research in neurosciences of the Society for Neurosciences and the Mexican policy NOM-062-ZOO-1999.
2.2. Behavioral procedures
Ovariectomized females were used for copulatory trials. Receptivity was induced with subcutaneous injections of estradiol benzoate (10μg; Sigma-Aldrich Mexico) followed by progesterone (2 mg; Sigma-Aldrich Mexico), both diluted in sesame oil and administered respectively 48 h and 4 h before mating. Sexual behavior was performed during the third phase of the inverted cycle.
Males were placed to copulate with sexually receptive females three days after the surgery (see below) and were removed of the arena after ejaculation. This procedure was repeated every two days for a total of five mating sessions. Following the last ejaculation (day 15 after surgery), males were anesthetized with an intraperitoneal injection of sodium pentobarbital (30 mg/ kg bw; Anesthesia, Smith Kline, Mexico). Nerve transections were re-examined in each animal, and they were subsequently sacrificed with a lethal dose of sodium pentobarbital (60 mg/ kg).
2.3. Surgery
Animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (30 mg/ kg bw; Anesthesia, Smith Kline, Mexico), then a longitudinal incision was made at the lower abdomen. The MPG was located in the lateral lobe of the prostate. Hypogastric and pelvic nerves were cut bilaterally, and a 1 cm section was extracted. In the Sham group, both nerves were manipulated but not cut. Finally, antibiotic (Enroxil 5%, 1mL / 20Kg SC) and analgesic (Meloxicam Syntex 2mg / Kg SC) was injected for 15 days. After males were euthanized, MPG was removed, and properly stored at -80°C until analysis.
2.4 Western blots
Tissue was homogenized with 50 μl buffer (1 M HEPES pH 7.9, 1 M KCl, 0.5 M EDTA pH 8, 100 mM PMSF pH 8 and Protease Inhibitor Cocktail), incubating 20 min at 4°C with 35 μl of 10% NP-40. Then the solution was centrifuged (1 min at 10,000 rpm, 4°C) and the liquid phase (cytoplasmic) separated, saved and frozen at -70°C. Chemicals were obtained from Sigma-Aldrich Chemical, Mexico. Protein concentration was determined by the Bradford method. 50 µg of protein for androgen and muscarinic and 25 µg for adrenergic and prolactin receptors were loaded on a gel of 10% polyacrylamide in the presence of SDS and reducing conditions and the samples were run at 100 V (Mini-Protean III, Bio-Rad). Then the proteins were transferred to a nitrocellulose membrane at 90 V for 2 h. The membrane was blocked with 5% milk (5%) diluted in 0.1% TBS-Tween for 1 h at room temperature and washed 3 times with a solution of TBS-Tween-20 (0.1%) for 5 min each. Then it was incubated overnight at 4°C with the first antibody against androgen receptor (C-19: 1:100), 1a-adrenergic receptor (H-136, sc-28982: 1:250), and M3 muscarinic receptor (H210, sc-9108: 1:500) from Santa Cruz Biotechnology and prolactin receptor (1:200) from Bioss (bs-6445R). On the next day, the membrane was washing 3 times and incubated with a secondary antibody (Goat anti-mouse 1:1000, Santa Cruz Biotechnology) for 1 h at room temperature. The bands were revealed by using the kit AP Conjugate substrate (170-6432, Bio-Rad) and densitometric analysis of the bands was performed by using the Kodak image station 440-CF with BioRad Molecular Image Gel DocTM XR+. GAPDH [FL 335, sc-25778: 1:500 (37 kDa)] was used the loading control.
2.5. Statistical analysis
The data for each test were compared using t student tests between naive and sexually expert subjects and one-way ANOVA for independent groups in denervated and SHAM animals followed by the post hoc Tukey test to compare groups of experimental animals against the corresponding control (n=6). Significant differences were inferred when p<0.05.
3.1 Effect of sexual behavior on expression receptors in MPG
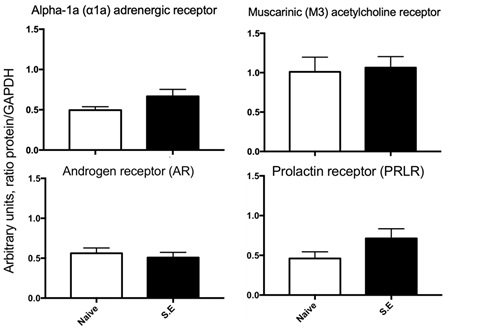
Analysis of copulatory parameters showed that males displayed the appropriate ejaculatory patterns. However, sexual behavior in the long-term did not affect the protein levels of any receptor analyzed in the ganglion (Fig 1).
3.2 Effect of denervation on the expression of receptors in MPG
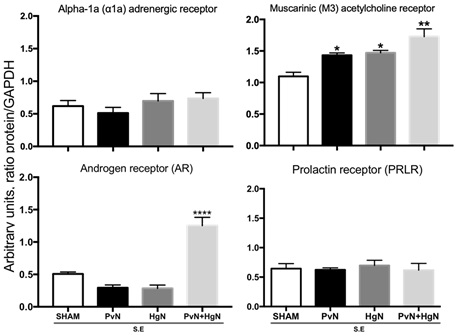
Receptors were analyzed in preganglionic denervated males and data show a clear increase in the protein of muscarinic and androgens receptors. The muscarinic receptor increased after the cut of pelvic or hypogastric nerves (p <0.05), being the increase higher after simultaneous denervations of the two nerves; the androgen receptor only increased with simultaneous denervations (p <0.05). Noradrenergic and prolactin receptors were not affected by denervations (fig.2).
Fig 1. Levels of adrenergic, muscarinic, androgen and prolactin receptors in the MPG of subjects without (naïve) or with sexual experience (SE). The figure shows that sexual behavior does not affect the levels of any receptor in the ganglion. Values represent the mean ± S.E.M. (n = 6). The data were compared using t student tests.
Fig 2. Levels of adrenergic, muscarinic, androgen and prolactin receptors in subjects with sexual experience (SE) and denervated. The figure shows that denervation increases the levels of muscarinic receptors after the lesion of either or both nerves, and androgen receptors just after the lesion of both nerves. Prolactin and adrenergic receptors were not affected by preganglionic denervation. Values represent the mean ± S.E.M. (n = 6). *, **p < 0.01, ***p < 0.001. One- way ANOVA for independent groups followed by the Tukey post hoc test were used.
The major pelvic ganglion (MPG) is attached to the dorsolateral prostate, contains adrenergic and cholinergic neurons and satellite and SIF cells, controls pelvic functions such as urination, defecation, erection and function of accessory glands such as the prostate1 and, in addition to containing receptors to these neurotransmitters, also express androgen12 and prolactin receptors (Unpublished in Prep). The execution of sexual behavior increases testosterone and prolactin in serum as well as its receptors in the prostate.10,11 Thus, the present hypothesis was that sexual behavior increases the expression of all these receptors, supported by the fact that sexual behavior increases MPG activity.9 The results show that sexual behavior did not affect the expression of any of the receptors analyzed, a situation that was unforeseen since at least an increase in androgen and prolactin receptors was expected. It is known that there is a relationship between acetylcholine and the androgen receptor because its addition promotes increased expression of the androgen receptor in the liver,13 but this effect was not observed in the MPG despite the fact that sexual behavior increases the activity of the ganglion.9 We found three possible explanations for this result: a) that it is not necessary to increase the expression of these receptors; b) that expression levels of these receptors have reached their maximum response as a result of multiple intromissions or ejaculations, or c) that their degradation is promoted when Mir206 is activated by the activation of AKT and Stat 3.13,14 All these proposals will be evaluated in future experiments. Although there is little information regarding the relationship between norepinephrine and androgen receptors, this relationship has been reported in the erection process.12,15 This neurotransmitter has a stimulatory effect mediated by the activation of alpha 2 receptors during the ejaculatory phase, without any change in the copulatory and precopulatory phase.16 Although this type of receptor was not analyzed, it is possible to assume that the analyzed alpha noradrenergic receptor behaves in the same way since it is a subtype of the noradrenergic receptor, but it can also be considered that this behavior does not exert its effect through this subtype of the receptor, so it would be appropriate to analyze alpha 2 adrenoreceptor under these experimental conditions.
Another aspect analyzed was the effect of denervation on the expression of these same receptors in subjects with sexual experience. The reason is because the prostate contains cholinergic and adrenergic fibers from neurons that are located in the MPG and its alteration induces the appearance of diseases in the gland 1,7,15,18, or prevent the development of cancer. In this sense, the deletion of beta-adrenergic receptors or the blockade of type 1 muscarinic receptors seems to prevent the metastasis or the development of prostate cancer.18,19 In previous works we observed histological changes in the prostate that correlate with a decrease in the expression of the receptor to androgens due to the effect of denervation, without altering the expression of noradrenergic, cholinergic and prolactin receptors.17 That is why the question analyzed in this work was whether these changes also correspond to changes in the expression of these same receptors in the ganglion. The results obtained showed an increase in the androgen receptor and the muscarinic receptor, without affecting the others (Fig. 2). The muscarinic receptor has the characteristic that its function and expression are regulated by the existence of a conserved triplet of amino acids (Asp-Arg-Tyr) located in the cytoplasmic domain, protecting it from degradation.20 Based on our results, it is suggested that the loss of the presynaptic fibers, and therefore of the neurotransmitter, results in an increase in the expression of the receptor, possibly by maintaining this triplet that prevents its degradation. Thus, the final contribution of this mechanism is to prolong the binding of the ligand to its receptor to avoid the early appearance of any disease in the prostate. Proposal is supported by the fact that a similar effect is observed with parasympathetic denervation of colon smooth muscle,21 indicating that cholinergic receptors respond rapidly to any change in neurotransmission.22
Finally, testosterone is a steroid hormone that promotes the expression of its own receptors10 and in denervated subjects, an increase in the expression levels of this receptor is observed (Fig. 2), but apparently this effect is not a consequence of the increase of testosterone in blood because presynaptic denervation blocks the testosterone increase in response to sexual behavior.17 For this reason, the increase in cholinergic and androgen receptors observed in the MPG seems to be regulated by the ANS rather than by the endocrine, that in turn means that ANS has a negative control over androgen receptor expression because both neurotransmitters are required to decrease the androgen and cholinergic receptor expression. These results are somehow similar to those reported by Kyi et al., 2017 in the way that PvN and HgN control cholinergic neurons but differ in that they observe a decrease in the expression of the receptors analyzed. The observed increase can be explained: 1) receptor analyzed was alpha 1, and 2) because this type of receptor came from subjects with sexual experience, notwithstanding what is a fact is that apparently these cholinergic neurons are regulated by these two nerves. Thus, the results obtained in this work coincide with the fact that the lack of innervation changes the expression of the receptors, in this case increasing the androgens and acetylcholine receptors from subjects with sexual experience, probably by increasing the synaptic strength of cholinergic neurons. This means that the fibers from PvN and HgN innervate cholinergic neurons that express the receptor to androgens23 and suggest that the loss of this nervous supply could be a mechanism that induces diseases in the prostate such as prostate cancer.
Based on the results obtained in this work, it is concluded that sexual behavior does not alter the expression of the receptors analyzed, but preganglionic denervation affects the expression of cholinergic and androgen receptors in the MPG, indicating that prostate diseases could be a consequence of changes in the expression of these types of receptors. Thus, prostate cancer treatments should consider at least these two types of receptors.
The author(s) declare that they have no competing interests.
Supported by Beca Conacyt No. 595375 (AMM), 595360 (VSZ), and Cuerpos Académicos de Neurociencias (UV-CA-28), and Academic Group of Neurochemistry (UV-CA-304).
1. Keas JR. Plasticity of pelvic autonomic ganglia and urogenital innervation. Int Rev Cytol 2006 248: 141-208.
2. Aldahmash A & Atteya M. Ganglionectomy in the adult male rat increases neuronal size and synaptic density in remaining contralateral major pelvic ganglion. Curr Neurobiol 2011 2: 5-15
3. Keast JR & Saunders RJ. Testosterone has potent, selective effects on the morphology of pelvic autonomic neurons which control the bladder, lower bowel and internal reproductive organs of the male rat. Neurosci 1998 85(2): 543-556
4. Eastham J, Stephenson C, Korstanje K, & Gillespie JI. The expression of β 3-adrenoceptor and muscarinic type 3 receptor immuno-reactivity in the major pelvic ganglion of the rat. Naunyn Schmiedebergs Arch Pharmacol 2015 388(7): 695-708.
5. Girard BM, Galli JR, Vizzard MA, & Parsons RL. Galanin expression in the mouse major pelvic ganglia during explant culture and following cavernous nerve transection. J Mol Neurosci 2012 48(3): 713-720.
6. Meusburger SM & Keast JR. Testosterone and nerve growth factor have distinct but interacting effects on structure and neurotransmitter expression of adult pelvic ganglion cells in vitro. Neurosci 2001 108(2): 331-340.
7. Diaz R, Garcia LI, Locia J, Silva M, Rodriguez S, Perez CA, Aranda-Abreu GE, Manzo J, Toledo R, & Hernandez, M. E. Histological modifications of the rat prostate following transection of somatic and autonomic nerves. Anais da Academia Brasileira de Ciências 2010 82(2): 397-404.
8. Nangle MR & Keast JR. Deafferentation and axotomy each cause neurturin-independent upregulation of c-Jun in rodent pelvic ganglia. Exp Neurol 2009 215(2): 271-280.
9. Fang J, Chung YW, & Clemens LG. Relation of Fos-IR expression in the pelvic ganglion to sexual behavior in laboratory rats. Behav Neurosci 2000 114(3): 543.
10. Hernandez ME, Soto-Cid A, Rojas F, Pascual LI, Aranda-Abreu GE, Toledo R, Garcia LI, Quintanar-Stephano, & Manzo J. Prostate response to prolactin in sexually active male rats. Reprod Biol Endocrin 2006 4(1): 28
11. Hernandez ME, Soto-Cid A, Aranda-Abreu GE, Díaz R, Rojas F, Garcia LI, Toledo R, & Manzo J. A study of the prostate, androgens and sexual activity of male rats. Reprod Biol Endocrin 2007 5(1): 11.
12. Schirar A, Chang C, & Rousseau JP. Localization of androgen receptor in nitric oxide synthase‐and vasoactive intestinal peptide‐containing neurons of the major pelvic ganglion innervating the rat penis. J Neuroendocrinol 1997 9(2): 141-150.
13. Nie H, Cao Q, Zhu L, Gong Y, Gu J, & He Z. Acetylcholine acts on androgen receptor to promote the migration and invasion but inhibit the apoptosis of human hepatocarcinoma. PloS one 2013 8(4): e61678
14. Chua, FY, & Adams BD. Androgen receptor and miR-206 regulation in prostate cancer. Transcription 2017 8(5): 313-327.
15. Traish A, Kim NN, Moreland RB, & Goldstein I. Role of alpha adrenergic receptors in erectile function. Int J Impot Res 2000 12(1): s48-s63
16. Snoeren, E. M. The role of adrenoceptors in the central nervous system in male and female rat sexual behavior. Eur J Pharmacol, 2015 753: 229-245.
17. Hernández-Aguilar ME, Serrano MK, Pérez F, Aranda-Abreu GE, Sanchez V, Mateos A, Manzo J, Rojas-Durán F, Cruz-Gomez Y, & Herrera-Covarrubias D. Quantification of neural and hormonal receptors at the prostate of long-term sexual behaving male rats after lesion of pelvic and hypogastric nerves. Physiol Behav 2020 222: 112915.
18. Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, & Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Sci 2013 341(6142): 1236361.
19. Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, Ittmann MM, & Rowley D. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 2008 14(23): 7593-7603.
20. Hamilton SE, Schlador ML, McKinnon LA, Chmelar RS, & Nathanson NM. Molecular mechanisms for the regulation of the expression and function of muscarinic acetylcholine receptors. J Physiol Paris 1998 92(3-4): 275-278.
21. Golder M, Burleigh DE, Belai A, Ghali L, Ashby D, Lunniss PJ, & Williams NS. Smooth muscle cholinergic denervation hypersensitivity in diverticular disease. The Lancet 2003 361(9373): 1945-1951.
22. Kyi C, Garcia V, & Schulz DJ. Impact of Decentralization on Cholinergic Neurotransmission in Neurons of Mouse Major Pelvic Ganglia. Faseb J 2017 31(1_supplement): 861-13.
23. Reilly CM, Stopper VS, & Mills TM. Androgens modulate the α‐adrenergic responsiveness of vascular smooth muscle in the corpus cavernosum. J Androl 1997 18(1): 26-31.
| Recibido: 12 de septiembre, 2020 | Aceptado: 05 de marzo, 2021 |
*Correspondence: Hernández-Aguilar María Elena. Av. Dr. Luis Castelazo Ayala s/n, col Industrial Las Ánimas, Xalapa, Veracruz, México. C.P. 91090, Tel: 52 (228) 8 418900 ext. 16307. E-mail: elenahernandez@uv.mx
Este es un artículo de libre acceso distribuido bajo los términos de la licencia de Creative Commons, (http://creamasal@unam.mxtivecommons.org/licenses/by-nc/3.0), que permite el uso no comercial, distribución y reproducción en algún medio, siempre que la obra original sea debidamente citada.