Male sexual behavior and prostate histology in a rat model of autism
- Inicio
- Comité Editorial
- Lineamientos
- Carta de Cesión de Derechos
- Información Legal
- Acerca de la Revista
- Bases de Datos
- Contacto
- ISSN 2007-3054
- Centro de Investigaciones Cerebrales
Universidad Veracruzana
Jorge Manzo1*, Genaro A. Coria-Avila1, Luis I. García1, Maria Elena Hernández1, Deissy Herrera-Covarrubias1, Rebeca Toledo1, Daniela Monje-Reyna1,2, Fidel Santamaria2
1Centro de Investigaciones Cerebrales, Universidad Veracruzana, Xalapa, Ver., Mexico, 2Department of Biology, The University of Texas at San Antonio, San Antonio, TX, USA.
Resumen/Abstract
Introduction
Results
Discussion
References
Correspondencia
En el presente trabajo estudiamos la conducta sexual y la histología de la próstata en un modelo de autismo en ratas macho. Los machos auitstas presentaron todos los parámetros conocidos de la copula pero la mayoría de ellos fuera de los niveles normales. Los patrones de conducta indicaron un decremento en el estado motivacional y una reducción en la potencia para la erección peneana y la eyaculación, sugiriendo alteraciones en vías centrales y autonómicas. La histología de la próstata reveló la presencia de displasia y anisocariosis, indicando un posible riesgo para el desarrollo de cáncer. Así, la conducta sexual y la fisiología reproductiva están presentes en ratas con autismo inducido, pero la ejecución se realiza con conductas inadecuadas y con diversas alteraciones que pueden resultar en infertilidad y cáncer de próstata.
Palabras clave: Erección peneana, Eyaculación, Cáncer de próstata, Infertilidad, Ácido valproico.
We studied the sexual behavior and prostate histology in a male rat model of autism. Autistic males displayed every known parameter of mating but most of them not at normal levels. Patterns indicated decreased motivational states and a reduced potency for penile erection and ejaculation, suggesting alterations in central and autonomic pathways. The histology of the prostate revealed dysplasia and anisokaryosis, indicating a possible risk of cancer development. While sexual behavior and reproductive physiology are present in rats with induced autism, the execution relies on inadequate behaviors and several alterations that could result in infertility and prostate cancer.
Keywords: Penile erection, Ejaculation, Prostate cancer, Infertility, Valproic acid.
1. Introduction
Autism is a spectrum of neurodevelopmental disorders with growing concern due to the high prevalence that is observed in children all over the world, with boys being more affected than girls in a 4:1 ratio. Autism has a wide spectrum of behavioral alterations; however, in most cases, it is not a life-threatening disorder. Thus, autistic children grow up to reach puberty and become sexually mature. Then, they face a new challenge, i.e., dealing with its own reproductive biology. Sexual education is a complex issue for adolescents with autism1 although most of them display reproductive responses such as menarche2 or normal plasma androgens,3 and in turn show sexual drive and accompanying behaviors, but they are displayed out of the social norms.4 The sexual behavior and physiology of reproduction in adolescents and adults with autism is still poorly understood, which affects the development of effective therapies and educational strategies. Thus, in this work we used a recognized rat model of autism to get further knowledge.
The laboratory rat has two remarkable features, it is an appropriate model of autism, and its sexual behavior and reproductive physiology have been thoroughly characterized.5-7 Two widely used rat models of autism is obtained by exposing embryos or pups to valproic acid (VPA). Rat embryos exposed to VPA on days 11.5 to 12.5 of gestation have a number of neuronal alterations that make them display autistic behaviors as newborns,8 being a useful procedure for the study of autism.9-12 The other VPA rat model of autism is obtained with a daily exposition to VPA from postnatal day 6 to day 12.13 While VPA induced autistic rats have been used to study behavioral, anatomical, and physiological aspects of autism there is no characterization of their sexual behavior and reproductive physiology. In this work, we studied sexual behavior in the postnatal VPA model of autistic male rats, as a first approach to characterize the physiological effects of autism in adolescence and adulthood.
The sexual behavior and reproduction of male rats have been thoroughly documented, and abundant literature dealing with its neural, endocrine, cellular, and molecular basis is available.5714-16 Behavioral patterns during copulation are identified as mount, intromission, and ejaculation, and are recorded as latencies for the first execution of each pattern and the frequency in which they are displayed in a single sexual bout. A male displays several mounts and intromissions until reaching ejaculation. After a refractory period, the male can display another group of patterns and a second ejaculation, and so on until reaching a state of exhaustion following several consecutive ejaculations. Quantification of these patterns have been useful to determine different aspects of reproduction in male rats after specific experimental procedures. We have reported that the transection of the visceral branch of the pelvic nerve alters sexual behavior due to changes in the processes of penile erection and ejaculation.17 In contrast, transection of the somatomotor branch did not affect sexual behavior but produced a decrease of the ejaculated seminal plug that reduces the fertility ratio of males, suggesting a reduction in the forceful tension required to expel the semen from the prostatic urethra to the female vagina.7 Thus, quantification of altered sexual behavior in male rats provides information on the underlying physiological changes. Therefore, here we used this behavior in order to propose some physiological status in the autistic male rat.
In addition to understanding how the nervous system affects sexual behavior in autistic individuals, it is important to understand its effects on the whole reproductive system. In this study we focused on the prostate gland as a first approach. The prostate is the largest sexual gland in the male rat reproductive tract that produces essential substances for the potency of sperm to fertilize eggs within the female reproductive tract. It is located at the proximal region of the urethra, the prostatic urethra, as a well-defined globular gland showing two distinctive regions, the dorsolateral and the ventral prostate, and the execution of sexual behavior leading to ejaculation has important effects on the endocrine responses of this gland.6 We have shown that minor endocrine alterations, as the modification in serum prolactin levels, which do not affect the execution of male sexual behavior, produced significant changes at the prostate epithelium that could account for triggering the development of hyperplasia or cancer;18 and prolactin is a key hormone in children with autism under pharmacological treatment. Risperidone is one of the most used medication in autistic children,19 and it is known that this treatment produces hyperprolactinemia.20 In this study we used VPA induced autistic rats to characterize their sexual patterns and to determine the histology of the prostate epithelium.
2. Materials and Methods
Two groups of sexually active Wistar rats (250-350 g/bw), two females and one male each, were allowed to live together for 10 days to ensure pregnancy.7 They were housed in plastic cages (60 x 40 x 22 cm) containing wood chip bedding and kept in a room maintained at 22 ± 2°C and under a 12:12 LD light schedule (lights on at 2200 h). Commercial pelleted rodent chow (Harlan, Mexico) and water were available ad libitum. After the initial 10 days, females were placed in individual cages for the rest of their pregnancy period. After birth, pup males were randomly assigned to the control group (Ctrl, n=7), or the VPA treated group (VPA, n=10). The day of birth was determined as postnatal day 0 (P0). Pups were continuously observed for proper development, and from P6 to P12 day received a daily i.p. injection of 1 ml of saline solution 0.9% (Ctrl group) or VPA dissolved in saline solution at a dose of 150 mg/kg. After injection, pups were returned to their mothers, weaned on P21, then housed with males of their own group. Each procedure was guided by the Society for Neuroscience Policy on the Use of Animals in Neuroscience Research and the Mexican Policy for the Use and Care of Lab Animals (NOM-062-ZOO-1999).
Control and VPA males were allowed to reach puberty (200-250 g/bw) to start the execution of sexual behavior with intact-developed ovariectomized receptive females. Receptivity was induced after females were injected subcutaneously with 10 µg of estradiol benzoate and 2 mg of progesterone, 48 and 4 h before tests, respectively. Once males reached adulthood, mating tests were conducted during the last third of the dark period. After 5 min adaptation of the male in a Plexiglas cylindrical arena (50 cm high x 50 cm diameter), a receptive female was introduced. The introduction of the female indicated the beginning of the test, the end of the test was after a 30 min period or after recording the male ejaculatory pattern. One copulatory series per animal was recorded twice a week until complete five mating tests. If ejaculation occurred, the couple was allowed to stay in the arena for both, to detect the extent of the refractory period and to determine the proportion of males that started a second copulatory series. Following the last mating test, 5 males chose at random in each group were anesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg bw). An abdominal midline longitudinal incision was made to reach the prostate, and tissue from the ventral (VP) and dorsolateral (DLP) regions of the gland were removed and processed for histology using the hematoxylin-eosin method.
The stain with hematoxilin-eosin started by immersing the prostate for 24 h in Helly fixative (Mercuric Chloride 5%, Potassium Dichromate 2.5%, Sodium Sulfate 1%, and Formalin 37%). Then the fixative was washed with tap water for 30 min. Dehydration was carried out by immersing the prostate in ethanol 70% (1 hr), 80% (1 hr), 96% (3 times, 2 hrs each), absolute ethanol (overnight), and absolute ethanol (2 times, 1 hr each). Clearing was done by immersing the prostate in xylene (3 times, 1 hr each). Both dehydration and clearing were carried out under continuous shaking. Then, the prostate was embedded in melted paraffin (2 times, 2 hr each at 57°C) and a block was done by using a microtome cassette. The paraffin block was sectioned on a Leica microtome (5 μm). Sections were placed on a mounting bath with gelatinized water at 52°C and tissues mounted on slides that were placed for 1 hr in an oven (~58°C). For staining, tissues were immersed in xylene (3 times, 5 min each), absolute ethanol/xylene 1:1 (5 min), ethanol 96% (3 min), iodine alcohol (5 min), sodium thiosulfate (10 min), tap water (2 min), Mayer's Hematoxilin (10 min), tap water (30 sec), acid ethanol (fast bath), tap water (10 sec), lithium carbonate (30 sec), tap water (10 sec), Eosin Y (4 fast baths), ethanol 96% (3 min), absolute ethanol (2 min), absolute ethanol/xylene 1:1 (2 min), and xylene (5 min). Finally, sections were coverslipped with undiluted Permount. Each slide had sections from just one region of the prostate, i.e., we obtained ventral slides and dorsolateral slides. The slides were observed in an Olympus Provis AX-70 microscope, and images obtained and analyzed with the Image-Pro Plus software. Measures obtained were the height of epithelial cells and the area of the alveoli.
The recording of copulatory movements guided the quantification of behavior. The number of mounts (NM) and number of intromissions (NI) represent their frequencies before ejaculation. The time elapsed from the introduction of the female with the male to the first mount or intromission was recorded as the latency of mount (LM) or latency of intromission (LI), respectively. If the first copulatory movement executed by the male was an intromission, then LM = LI. The time elapsed from the first intromission to the ejaculation was recorded as the latency of ejaculation. A proportion of intromission (hits) was obtained by computing NI/(NM+NI), the result is known as the Hit Rate parameter of copulatory behavior and reflects the capacity of the male to intromit and, therefore, to have erection of the penis.21 According to the experimental design, the graphs and statistical analysis were done with Prism 8 (GraphPad Software, Inc.) by using the Nested t test procedure with significant comparisons when p<0.05.
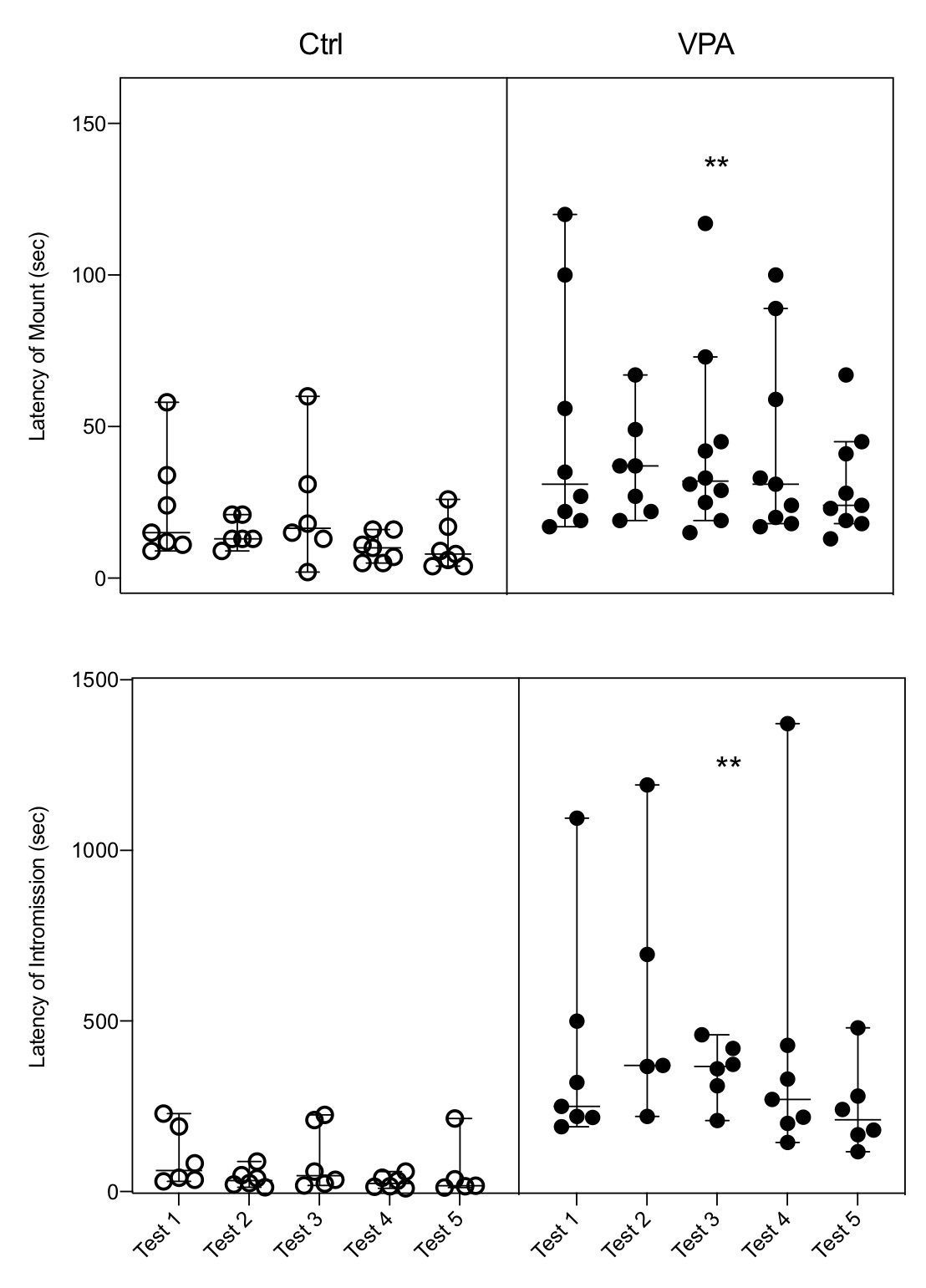
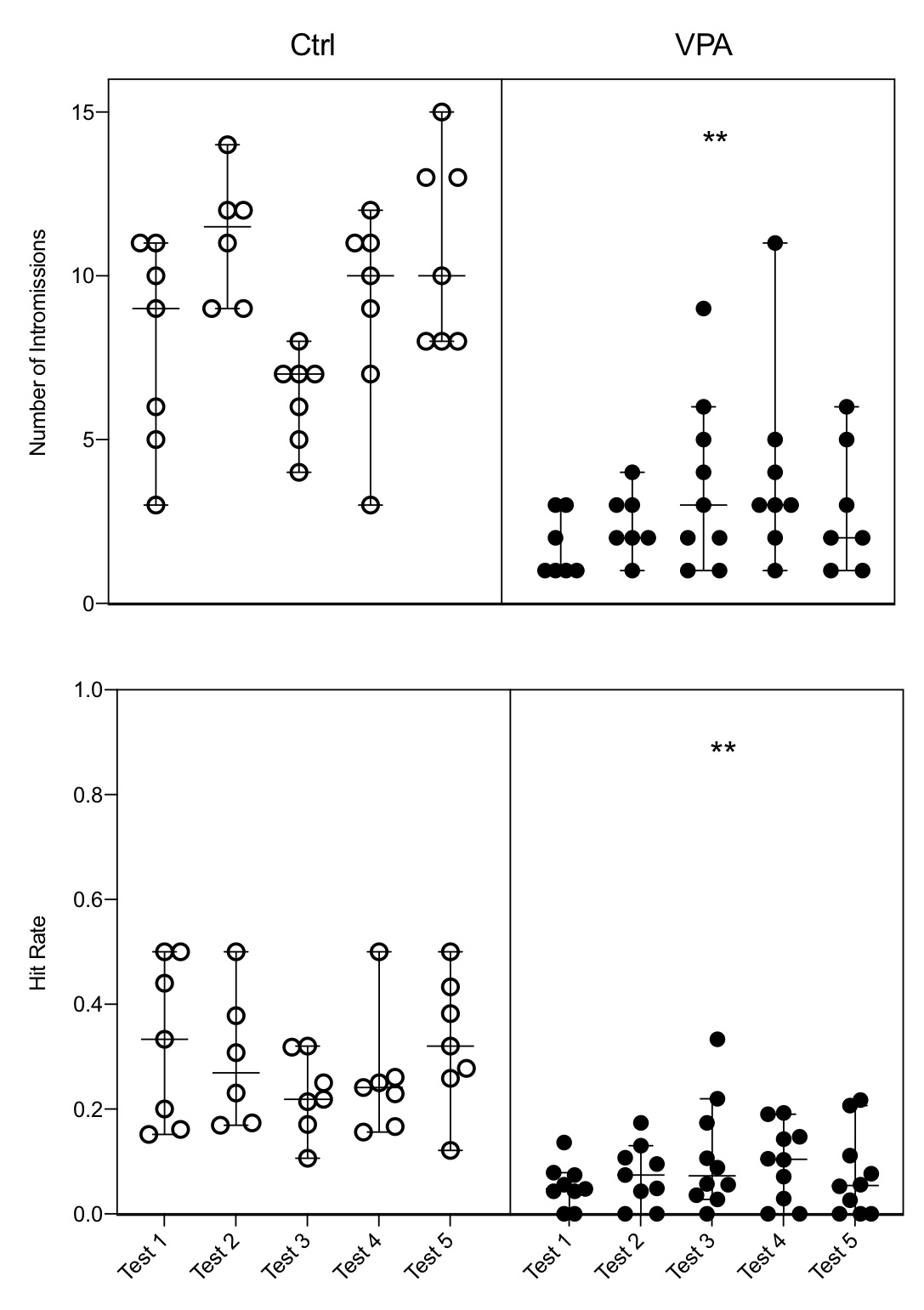
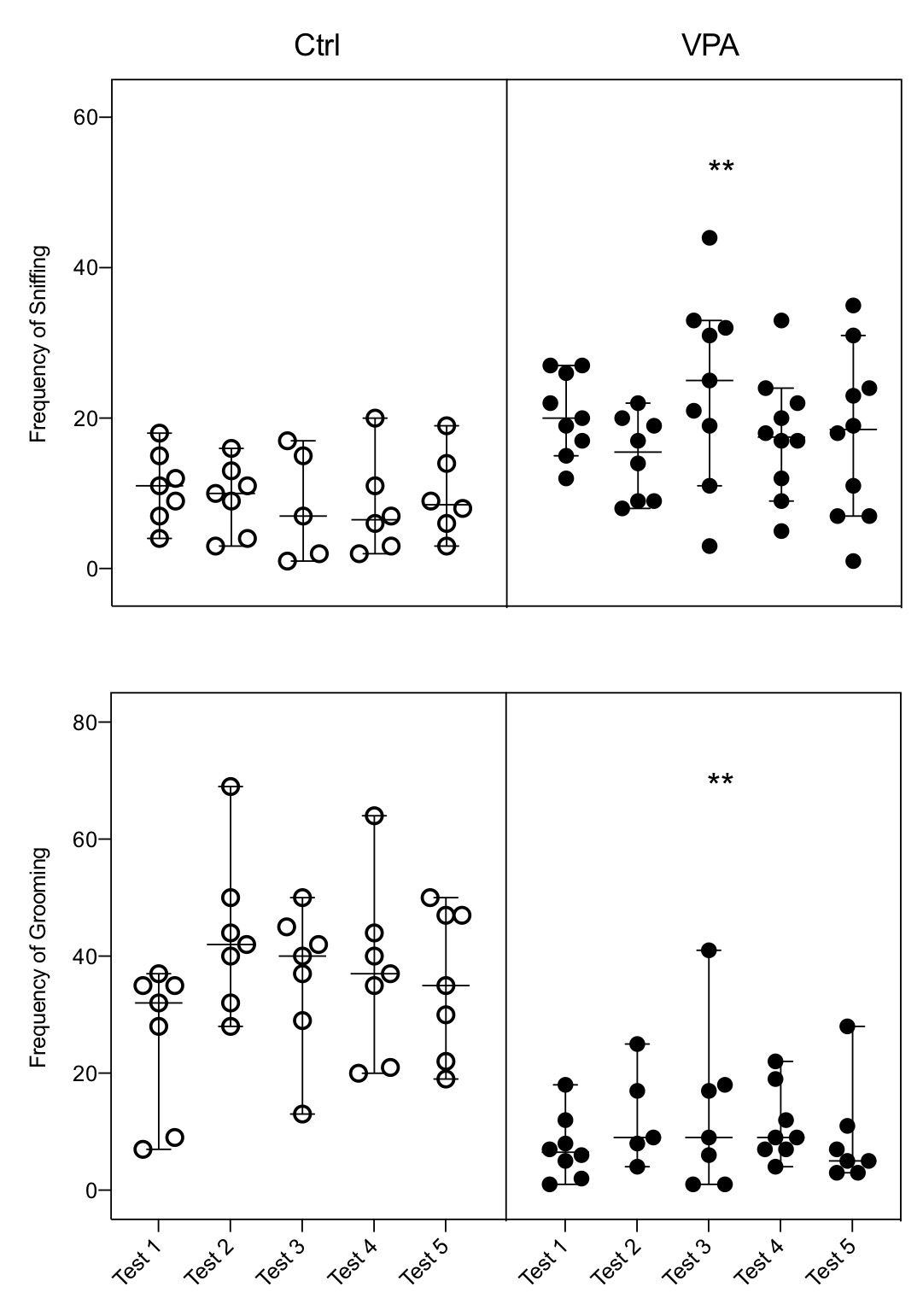
Results showed that male rats with induced autism display every known parameter of sexual behavior. However, quantification of behavioral displays indicates that some of them are not expressed in the level measured for control males. Hence, data indicated that both the latency of mount and latency of intromissions were increased (Fig. 1); the number of mounts had no change; and the number of intromissions and the hit rate were reduced (Fig. 2). The ejaculation pattern in the five tests was observed in 85% of control males and in 20% of autistic males, and these numbers precluded a proper analysis of the latency of this parameter; from these ejaculating males 71% of controls started a second ejaculatory series, while only 10% of autistic males started it. Adjunct behaviors were also modified in the autistic group, i.e., sniffing was increased, and grooming was reduced (Fig. 3).
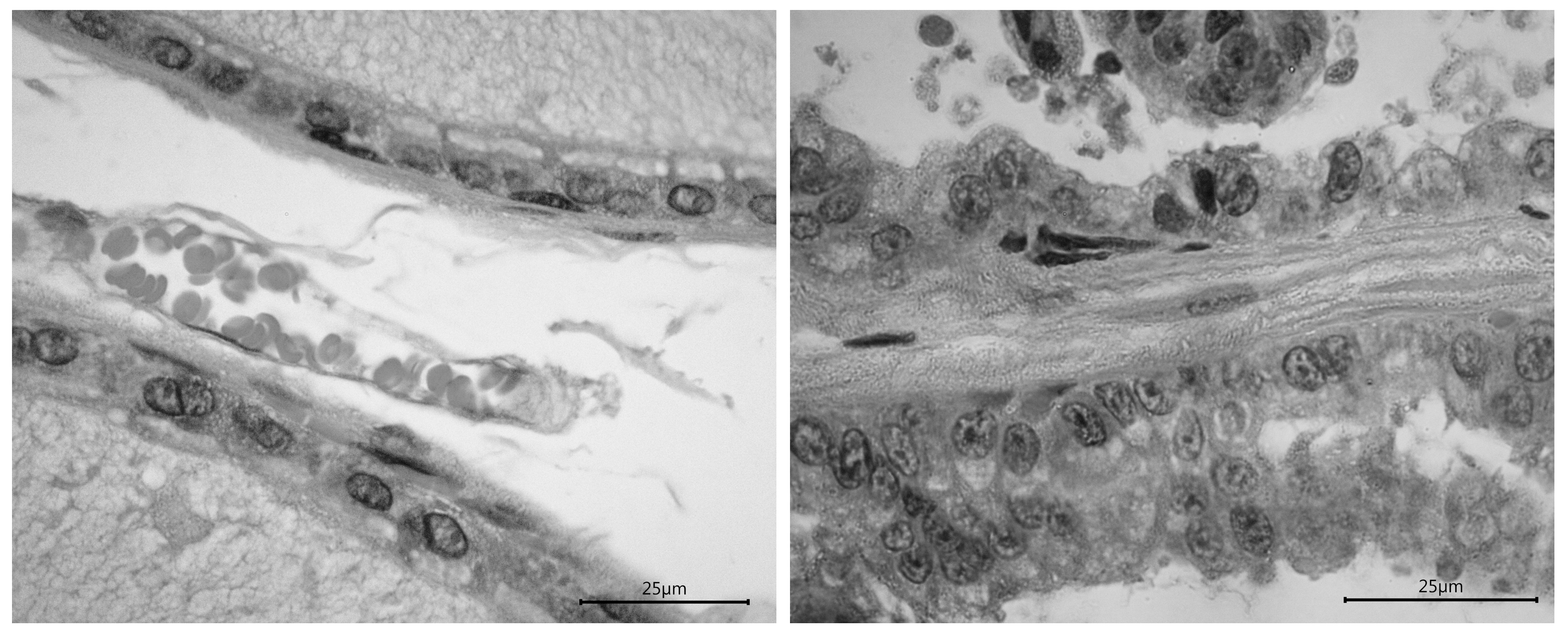
The histological analysis showed that the dorsolateral prostate had a cubic epithelium in each control male, while 3 out of 5 autistic males had an abnormal size or anisocytosis. The image analysis also revealed an even interstitial space with collagen in every control male, but in 2 out of 5 autistic males the space was compressed and showed mononuclear cells; the nuclei had an homogeneous size with a polarity basal location in control males, but in every autistic male the polarity was lost and in two males nuclei showed an abnormal size or anisokaryosis. The ventrolateral prostate showed similar alterations(Fig. 4).
Figure 1. The latencies of mount and intromission showed a significant Nested increase in the autistic group (VPA) as compared with the control group (Ctrl), and the increase is observed in each of the five tests of the VPA group. Each bar represents the median and the 95% confidence level per test in each group; circles on bars represents the value obtained per animal, indicating how many males executed the behavior per test. LM: F(1,74)=21.24; LI: F(1,58)=32.23; ** = p<0.01.
Figure 2. The number of intromissions and the hit rate showed a significant Nested decrease in the autistic group (VPA) as compared with the control group (Ctrl), and the decrease is observed in each of the five tests of the VPA group. Each bar represents the median and the 95% confidence level per test in each group; circles on bars represents the value obtained per animal, indicating how many males executed the behavior per test. NI: F(1,8)=37.94; HR: F(1,80)=87.61; ** = p<0.01.
Figure 3. Alteration of adjunct behaviors. The frequency of sniffing (FS) showed a significant Nested increase in the autistic group (VPA) as compared with the control group (Ctrl), while the frequency of grooming (FG) showed a significant decrease, and the respective change in both behaviors were observed in each of the five tests of the VPA group. Each bar represents the median and the 95% confidence level per test in each group; circles on bars represents the value obtained per animal, indicating how many males executed the behavior per test. FS: F(1,8)=25.41; FG: F(1,8)=70.29; ** = p<0.01.
Figure 4. Representative images of the dorsolateral prostate in a control male (left photo) and an autistic male (right photo). The images reveal the differences between groups; it is remarkable the alteration in the epithelium and nuclei. Calibration bars = 25 µm.
The sensory processing behaviors related to sniffing that we observed are consistent with the reported behavior of autistic patients. Our observation that autistic rats have increased sniffing during sexual behavior is consistent with a reduced sensitivity in olfactory tests of male children with autism,22,23 that are extended into adult life.24 Olfactory processing is an important issue in triggering sexual responses. Sexual odors from the females activate the piriform cortex,25 that is further activated during sexual behavior,26 and that shows altered cytoarchitecture in autism.27 Hence, it seems that the used autistic model rat has also alterations in the olfactory pathway that explain the increase in sniffing. We also suggest that the decreased olfactory sensitivity in autistic rats results in the reduction of the sexual motivation state of the male. This reduced motivation is observed in the increased latency of both mounts and intromissions. It is known that sexual motivation is under the control of several brain structures, steroid hormones, and neurotransmitters,28 making complex circuits that might be altered in the autistic rats. The social motivation theory of autism29 states that disruption of motivational mechanisms is based in brain structures as the amygdala, the ventral striatum, and prefrontal cortex. Thus, our data suggest that the VPA induced rat model of autism is a good model to test both the physiological and anatomical correlates of the social motivational theory of autism and the overall physiology of sexual behavior.
The increased time in the latency and reduction of the number of intromissions indicate a lower potency for penile erection. Similar results of these parameters were observed previously in male rats after transection of the viscerocutaneous branch of the pelvic nerve.17 Furthermore, it has been suggested that reduced values of the hit rate parameter are consequence of treatments that alters the erectile potential or penile sensitivity.21 The physiology of penile erection relies on a complex system that involve peripheral autonomic and central nervous control as well as endocrine processes, but it is completely unknown the function of each system in autism. It is known, however, that autonomic impairments in cardiovascular physiology are found in autism,30 and that penile erection is under autonomic control of vascular events in the penile body.31 Thus, analyses of our behavioral data suggest that some autonomic functions could be altered in the autistic rat.
Ejaculation is a process that activates neural circuits involved in dopaminergic and serotonergic pathways, 32,33 as well as some key areas as the amygdala and the cerebellum.34,35 All these pathways and areas are altered in autism.36-39 Thus, the observed reduced proportion of autistic males reaching ejaculation suggests that they have alterations in these structures. In a previous study we showed that prenatal exposure to VPA alters androgen receptors in the cerebellum of male rats.12 In combination with present study, the results further support the hypothesis that the neural alterations in the VPA rat model of autism are on the regions that regulates different aspects of male reproduction. On the other hand, penile stimuli by intromissions or ejaculation triggers the genital grooming behavior that seems to be integrated in a copulatory motor program.40 This adjunct behavior was reduced in the autistic rats, indicating that its physiology is also altered in autism. Therefore, it is concluded that there are different misfunctions in several aspects of the masculine sexual behavior in autism that suggest a high tendency for infertility.
In addition to behavior, the histology of the autistic prostate showed cellular cytoarchitecture conditions, such as dysplasia and anisokaryosis, that are consistent with cancerous tissue.41 Previously we reported similar cellular changes in the prostate induced by testosterone treatment.42 We have also shown that the execution of sexual behavior produces an increase in both serum levels of testosterone and androgen receptors in the prostate.43 Furthermore, we also showed that similar effects at the prostate are produced after lesions of the autonomic nerves that supply the gland.44 All these data strongly suggest that the prostate is an autism-sensitive gland for the development of cancer and its alteration could add to the behavioral tendency for infertility. Although a number of studies have reported correlations between autism and cancer,45-47 to our knowledge, this is the first study indicating a high risk for the development of prostate cancer in autistic males. Therefore, as far as new information become available, the analysis of prostate health in sexually mature men with autism should be an area of particular scrutiny.
None.
This study was supported by a 2017 Collaborative Research Project from the University of Texas System and the Consejo Nacional de Ciencia y Tecnología de México (ConTex Program) to JM and FS.
1. Holmes LG, Himle MB, Sewell KK, Carbone PS, Strassberg DS, Murphy NA. Addressing Sexuality in Youth with Autism Spectrum Disorders: Current Pediatric Practices and Barriers. J Dev Behav Pediatrics. 2014;35(3):172.
2. Knickmeyer RC, Wheelwright S, Hoekstra R, Baron-Cohen S. Age of menarche in females with autism spectrum conditions. Dev Med Child Neurol. 2006;48(12):1007-1008.
3. Tordjman S, Anderson GM, McBride AP, Hertzig ME, Snow ME, Hall LM, Ferrari P, Cohen DJ. Plasma androgens in autism. J Autism Dev Disord. 1995;25(3):295-304.
4. Coleman M, Gillberg C. The Autisms. Oxford University Press; 2012.
5. Manzo J, Carrillo P, Coria-Avila GA, Garcia LI. The sexual cerebellum. En: Komisaruk BR and G Gonzalez-Mariscal, Behavioral neuroendocrinology. CRC Press 2017:103-112.
6. Hernandez ME, Soto-Cid A, Aranda-Abreu GE, Diaz R, Rojas F, Garcia LI, Toledo R, Manzo J. A study of the prostate, androgens and sexual activity of male rats. Reprod Biol Endocrin. 2007;5(1):11.
7. Manzo J, Vazquez MI, Cruz MR, Hernandez ME, Carrillo P, Pacheco P. Fertility ratio in male rats Effects after denervation of two pelvic floor muscles. Physiology Behav. 2000;68(5):611-618.
8. Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: Developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370(2):247-261..
9. Oyabu A, Narita M, Tashiro Y. The effects of prenatal exposure to valproic acid on the initial development of serotonergic neurons. Int J Dev Neurosci. 2013;31(3):202-208.
10. Mychasiuk R, Richards S, Nakahashi A, Kolb B, Gibb R. Effects of Rat Prenatal Exposure to Valproic Acid on Behaviour and Neuro-Anatomy. Dev Neurosci-basel. 2012;34(2-3):268-276.
11. Fueta Y, Sekino Y, Yoshida S, Kanda Y, Ueno S. Prenatal exposure to valproic acid alters the development of excitability in the postnatal rat hippocampus. Neurotoxicology. 2018.
12. Perez-Pouchoulen M, Miquel M, Saft P, Brug B, Toledo R, Hernandez ME, Manzo J. Prenatal exposure to sodium valproate alters androgen receptor expression in the developing cerebellum in a region and age specific manner in male and female rats. Int J Dev Neurosci. 2016;53:46-52.
13. Reynolds S, Millette A, Devine DP. Sensory and Motor Characterization in the Postnatal Valproate Rat Model of Autism. Dev Neurosci-basel. 2012;34(2-3):258-267.
14. Manzo J, Miquel M, Toledo R, Mayor-Mar JA, Garcia LI, Aranda-Abreu GE, Caba M, Hernandez ME. Fos expression at the cerebellum following non-contact arousal and mating behavior in male rats. Physiol Behav. 2008;93(1-2):357-363.
15. McGinnis M, Dreifuss R. Evidence for a role of testosterone-androgen receptor interactions in mediating masculine sexual behavior in male rats. Endocrinology. 1989;124(2):618-626.
16. Sachs BD, Barfield RJ. Temporal patterning of sexual behavior in the male rat. J Comp Physiol Psych. 1970;73(3):359.
17. Lucio RA, Manzo J, Martínez-Gómez M, Sachs BD, Pacheco P. Participation of pelvic nerve branches in male rat copulatory behavior. Physiology Behav. 1994;55(2):241-246.
18. Hernandez M, Soto-Cid A, Rojas F, Pascual LI, Aranda-Abreu GE, Toledo R, Garcia LI, Quintanar-Stephano A, Manzo J. Prostate response to prolactin in sexually active male rats. Reprod Biol Endocrin. 2006;4(1):1-12.
19. DeFilippis M, Wagner K. Treatment of Autism Spectrum Disorder in Children and Adolescents. Psychopharmacol Bull. 2016;46(2):18-41.
20. Anderson GM, Scahill L, McCracken JT, McDougle CJ, Aman MG, Tierney E, Arnold LE, Martin A, Katsovich L, Posey DJ, Shah B, Vitiello B. Effects of Short- and Long-Term Risperidone Treatment on Prolactin Levels in Children with Autism. Biol Psychiat. 2007;61(4):545-550.
21. Meisel RL, Sachs BD. The physiology of male sexual behavior. En: Knobil E and JD Neill, The physiology of reproduction. Raven Press 1994:3-105.
22. Dudova I, Vodicka J, Havlovicova M, Sedlacek Z, Urbanek T, Hrdlicka M. Odor detection threshold, but not odor identification, is impaired in children with autism. Eur Child Adoles Psy. 2011;20(7):333-340.
23. Muratori F, Tonacci A, Billeci L, Catalucci Tiziana, Igliozzi R. Olfactory Processing in Male Children with Autism: Atypical Odor Threshold and Identification. J Autism Dev Disord. 2017;47(10):3243-3251.
24. Wicker B, Monfardini E, Royet J-P. Olfactory processing in adults with autism spectrum disorders. Mol Autism. 2016;7(1):4.
25. Pfaus JG, Tse TLM, Werk CM, Chanda ML, Leblonde A. Enhanced synaptic responses in the piriform cortex associated with sexual stimulation in the male rat. Neuroscience. 2009;164(4):1422-1430.
26. Robertson GS, Pfaus JG, Atkinson LJ, Matsumura H, Phillips AG, Fibiger HC. Sexual behavior increases c-fos expression in the forebrain of the male rat. Brain Res. 1991;564(2):352-357.
27. Menassa DA, Sloan C, Chance SA. Primary olfactory cortex in autism and epilepsy: increased glial cells in autism. Brain Pathol. 2017;27(4):437-448.
28. Ågmo A. Male rat sexual behavior. Brain Res Protoc. 1997;1(2):203-209.
29. Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239.
30. Bujnakova I, Ondrejka I, Mestanik M, Visnovcova Z, Mestanikova A, Hrtanek I, Fleskova D, Calkovska A, Tonhajzerova I. Autism spectrum disorder is associated with autonomic underarousal. Physiol Res. 2016;65(Supplementum 5):S673-S682.
31. Giuliano F. Neurophysiology of Erection and Ejaculation. J Sex Medicine. 2011;8(s4):310-315.
32. Peeters M, Giuliano F. Central neurophysiology and dopaminergic control of ejaculation. Neurosci Biobehav Rev. 2008;32(3):438-453.
33. Giuliano F, Clément P. Physiology of Ejaculation: Emphasis on Serotonergic Control. Eur Urol. 2005;48(3):408-417.
34. Kondo Y. Lesions of the medial amygdala produce severe impairment of copulatory behavior in sexually inexperienced male rats. Physiol Behav. 1992;51(5):939-943.
35. Garcia-Martinez R, Miquel M, Garcia LI, Coria-Avila GA, Perez CA, Aranda-Abreu G, Toledo R, Hernandez ME, Manzo J. Multiunit Recording of the Cerebellar Cortex, Inferior Olive, and Fastigial Nucleus During Copulation in Naive and Sexually Experienced Male Rats. Cerebellum. 2010;9(1).
36. Muller CL, Anacker AMJ, Veenstra-VanderWeele J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience. 2016;321:24-41.
37. Pavăl D. A Dopamine Hypothesis of Autism Spectrum Disorder. Dev Neurosci-basel. 2017;39(5):355-360.
38. Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SCR. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24(3):355-364.
39. Stoodley CJ. The Cerebellum and Neurodevelopmental Disorders. Cerebellum. 2016;15(1):34-37.
40. Sachs BD, Clark JT, Molloy AG, Bitran D, Holmes GM. Relation of autogrooming to sexual behavior in male rats. Physiology Behav. 1988;43(5):637-643.
41. Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004;4(9):nrc1430.
42. Herrera-Covarrubias D, Tecamachaltzi-Silvaran M, Barradas-Moctezuma M, Rosales-Raya JB, Manzo J, Garcia LI, Aranda-Abreu GE, Ismail N, Coria-Avila GA, Hernandez ME. Effect of copulation on potentially precancerous prostate lesions, serum testosterone and prolactin levels in rats. Exp Oncol. 2016;38(2):73-79.
43. Hernandez M, Soto-Cid A, Aranda-Abreu GE, Diaz R, Rojas F, Garcia LI, Toledo R, Manzo J. A study of the prostate, androgens and sexual activity of male rats. Reprod Biol Endocrin. 2007;5(1):11.
44. Diaz R, Garcia LI, Locia J, Silva M, Rodriguez S, Perez CA, Aranda-Abreu GE, Manzo J, Toledo R, Hernandez ME. Histological modifications of the rat prostate following transection of somatic and autonomic nerves. Anais da Academia Brasileira de Ciências. 2010;82(2):397-404.
45. Crespi B. Autism and cancer risk. Autism Res. 2011;4(4):302-310.
46. Wen Y, Herbert MR. Connecting the dots: Overlaps between autism and cancer suggest possible common mechanisms regarding signaling pathways related to metabolic alterations. Med Hypotheses. 2017;103:118-123.
47. Darbro BW, Singh R, Zimmerman BM, Mahajan VB, Bassuk AG. Autism Linked to Increased Oncogene Mutations but Decreased Cancer Rate. Plos One. 2016;11(3):e0149041.
| Recibido: 09 de agosto de 2019 | Aceptado: 28 septiembre de 2019 |
Corresponding Author at: Centro de Investigaciones Cerebrales. Universidad Veracruzana. Xalapa, Ver. Mexico. C.P. 91010, Tel: 52 (228) 8 418900 ext. 16309. E-mail: jmanzo@uv.mx
Este es un artículo de libre acceso distribuido bajo los términos de la licencia de Creative Commons, (http://creativecommons.org/licenses/by-nc/3.0), que permite el uso no comercial, distribución y reproducción en algún medio, siempre que la obra original sea debidamente citada.