Neuropsychological subtypes of schizophrenia and prefrontal circuits
- Inicio
- Comité Editorial
- Lineamientos
- Carta de Cesión de Derechos
- Información Legal
- Acerca de la Revista
- Bases de Datos
- Contacto
- ISSN 2007-3054
- Centro de Investigaciones Cerebrales
Universidad Veracruzana
Uribe Ezequiel
Centro de Biofísica y Neurociencias. Laboratorio de Neurobiología Conductual. Escuela de Ciencias Biomédicas, Universidad de Carabobo, Valencia, Venezuela. Programa Doctoral en Ciencias Médicas, Universidad de Carabobo, Valencia, Venezuela.
Abstract/Resumen
Introducción
Conclusiones
Agradecimientos
Referencias
Correspondencia
Schizophrenia is a neurodegenerative and disabling psychiatric illness which its physiopathology is still unknown. The spectrum of symptoms is widely variable among patients; which suggest that some variants of the disease affect different brain circuits, generating specific neuropsychological deficits. Thus, we propose that every subtype of schizophrenia could arise from the dysfunction of specific prefrontal circuits, inducing different clinical symptoms and neuropsychological deficits.
Key words: Prefrontal cortex, Hippocampus, Working memory, Disexecutive syndrome, Paranoid delusions, Catatonia.
La esquizofrenia es una enfermedad psiquiátrica neurodegenerativa y discapacitante cuya fisiopatología es aún desconocida. El espectro de síntomas es ampliamente variable entre un paciente y otro, lo cual sugiere que algunas variantes de la enfermedad afectan diferentes circuitos cerebrales, generando deficits neuropsicológicos específicos. Por lo tanto, se propone que cada subtipo de esquizofrenia pudiera provenir de la disfunción de circuitos prefrontales específicos, incluyendo diferentes síntomas clínicos y deficits neuropsicológicos.
Palabras clave: Corteza prefrontal, Hipocampo, Memoria de trabajo, Disejecutividad, Delirios paranoides, Catatonia.
Schizophrenia is a disabling disorder which affects 1% of the world population, involving several brain areas and mental functions like thought, mood, perception and cognition.1 Currently, there exist 5 subtypes of schizophrenia2 i.e. paranoid, catatonic, hebephrenic, simple and undifferentiated, following specific diagnostic criteria of Diagnostic and Statistical Manual of Mental Disorders IV Text Revision (DSMIV TR, Table 1). Scientific research has made remarkable efforts to establish the neurobiological basis of this condition, achieving a better comprehension of this brain disorder. Nevertheless, this knowledge hasn’t reached yet a direct impact over treatment, course and prognosis of schizophrenia. In addition, once the illness begins, there are no clinical tools to efficiently predict the pattern of cognitive and brain damage, elements deeply different from one patient to other. This situation could come from the fact that, almost all the disease related scientific publications express their results without characterize the specific subtype of schizophrenia, moreover the tendency to ignore negative results worsens this situation because both positive and negative results are relevant to show if specific neuronal networks or brain circuits are affected in one subtype of schizophrenia and not in another. Even worse, despite DMS-V maintains the general diagnosis criteria for schizophrenia (table 2), the clinical criterion for the diagnosis of subtypes were removed, due to the fact that almost all patients developed criteria for more than one subtype of schizophrenia during the illness course, and generally are less used by psychiatrists.3
Table 1. Diagnostic Criteria for subtypes of Schizophrenia. DSM IV TR
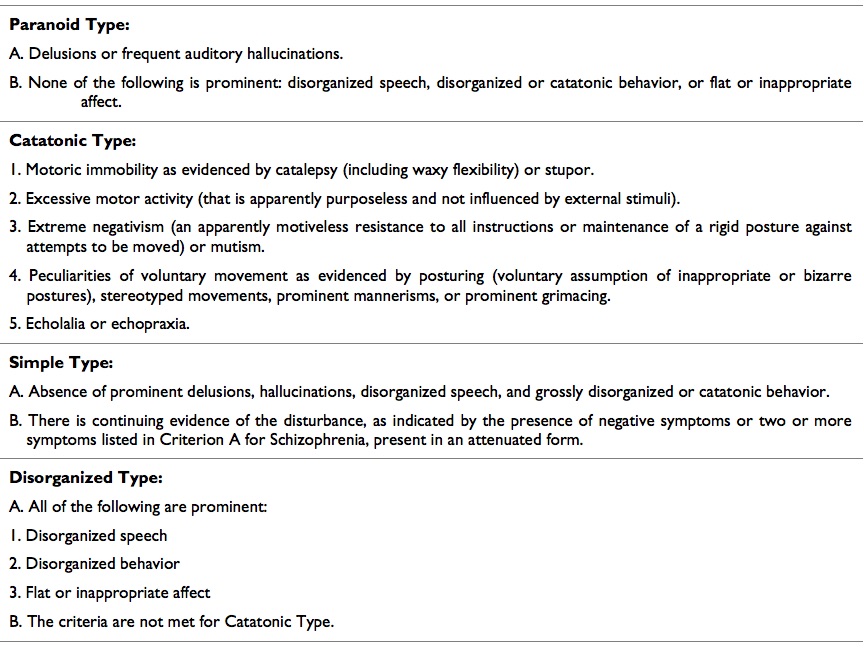
Table 2. Diagnostic Criteria for Schizophrenia. DSM V

Schizophrenic patients have marked differences in the clinical symptoms, as well as an extremely variable pattern of brain damage, involving both, cortical and subcortical areas. However, recently it was reported that the frontostriatal circuit plays an important role in schizophrenia.4 The frontal lobe shows the most extensive connectivity of the brain, with five main circuits: motor, oculomotor, dorsolateral, orbitofrontal and anterior.5 The last three circuits present a close association with schizophrenia because, their dysfunction produce multiple neuropsychiatric syndromes that mimicking many of the symptoms caused in every subtype of the disease. Therefore, we propose that every subtypes of schizophrenia comes from a different prefrontal circuit, generating a particular pattern of neurocognitive impairment, which, once delimited, could be effectively approachable through neuropsychological assessment.
2. Paranoid Schizophrenia and orbitofrontal circuit
Paranoid schizophrenia is the most common subtype of the disease and frequently presents the better prognosis.6 Delusions and auditory hallucinations are typical; especially during the psychotic episodes that in general terms, require hospitalization. The prodromal phase involves at least six months of social isolation and cannabis use previous to the onset.7 Appropriate antipsychotic treatment has been related with a reduction of relapses; due to the fact that every new psychotic crisis aggravates the long term prognosis and social functionality.8
Paranoia is an abnormality of thought content and come from a judgment defect. Personality and mood are not permanently involved, but contribute with it in each relapse. The prognosis of this subtype of schizophrenia is favorable.9
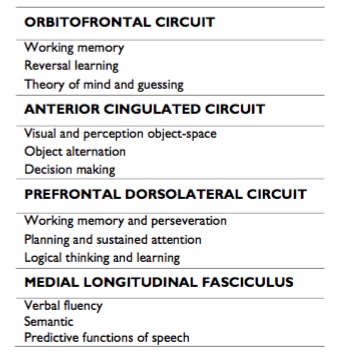
The orbitofrontal circuit connects the frontal monitory system with limbic structures and any dysfunction in these brain region generates changes in personality, disinhibition and emotional lability, showing an inappropriate interpretation of social elements,5 as well as a mistaken performance during the Wisconsin Test10 (for evaluate working memory and perseveration), both elements present in paranoid schizophrenia.11 Auditory hallucinations and paranoid delusions are developed in frontotemporal dementia, involving a degeneration of this brain circuit.12 A medial division of the orbitofrontal circuit has been described, which begins from the medial orbital gyrus (11 Broadmann area), and connects to nucleus accumbens, medial globus pallidus, temporal lobe and mediodorsal thalamic nucleus, returning to the medial orbital gyrus. The medial orbitofrontal cortex of patients with paranoid schizophrenia reveals activation abnormalities in functional Magnetic Resonance Imaging (fMRI) during the presentation of images of sad, furious and cheerful faces;13 furthermore, this region shows a significant reduction of grey matter also associated to a volume decrease of medial temporal gyrus14 and persistent auditory hallucinations.15 Other neurologic conditions like Persecutory Delusions Following Traumatic Brain Injury (PDFTBI) and Capgras syndrome involve abnormalities in medial frontal cortex and anterior temporal poles, also present emotional faces recognition difficulty and paranoid delusions.16 The medial orbitofrontal circuit is related to guessing task (more than planning task), in which there is no rational basis to choose one answer over another, forcing the subject to make associations,17 which is the mental process recently involved in delusion formations.18Thus, paranoid schizophrenia could be neuropsychologicaly evaluated with specific tests to evaluate the medial orbitofrontal cortex. This scenario explains why these patients show a normal performance during intelligence, language, visospatial, attention and executive task;19 even in reward tasks,20which are related to the lateral portion of the orbitofrontal cortex.17
The reversal learning is also abnormal in paranoid schizophrenia;21 this deficit is more evident when reward is not present in the task.22 However, the link among orbitofrontal cortex, paranoid schizophrenia and reversal learning is poor, mainly because those publication do not report the sub type of the disease. Clearly, patients with orbitofrontal cortex lesions show a similar behavior and paranoid delusion of those with paranoid schizophrenia,23 but different than patients with prefrontodorsolateral lesions,24 whose behavior is successful linked with the simple schizophrenia sub type. Theory of mind (ToM) is another function of orbitofrontal area, which is abnormal in paranoid schizophrenia,25 which consist in the capacity of a person to infer the feelings and thoughts of a conspecific. It is compose for emotional and cognitives components, being the emotional one, more affected in orbitofrontal lesion, producing hippomaniac and manic states with a remarkable social dysfunction.26 Paranoid delusions, mayor criteria of paranoid schizophrenia, have been linked to a manic tendency and to an increment of the self-proportion.27
3. Catatonic schizophrenia and anterior cingulated circuit
Catatonia was described the first time by Karl Kahlbaum in 1870, as a medical entity different from schizophrenia; years later (1971) it was integrated to the psychiatry by Emil Kraepelin as a subtype of the disease,28 until the publication of DSM IV, being recently removed as schizophrenia subtype and becoming in a sign of severity of many psychiatric disorders by the DSM V,29 mainly because catatonia is more prevalent in mood disorders.30 Almost all European psychiatry joined Jasper’s definition of catatonia: “somewhere between the neurological phenomena, seen as disturbances of the motor apparatus, and the psychological phenomena, seen as the sequel of psychic abnormality with the motor apparatus intact, lie the psychic motor phenomena, which we register without being able to comprehend them satisfactorily one way or the other”.31This definition excludes a possible biologically base for catatonia however, the first definition of catatonia by Kahlbaum was clinically sophisticated, describing the akinetic form like a state of rigid immobility, fixed gaze without winking and rigidity. Aggressive form of catatonia, described almost fifty years later by Kretschmer, involves extreme aggressiveness, hyperkinesia and orallity.32 As we can see, since the first definitions, catatonia had diffuse conceptual limits, therefore DSM V establish formal diagnosis criteria, in which three (3) or more of the following must be present: catalepsy, waxy flexibility, stupor, agitation, mutism, extreme negativism, posturing, mannerisms, stereotypes, grimacing, echolalia and echopraxia.29 Catatonic schizophrenia could, episodically, show any of the diagnosis criteria for other subtypes of the disease, but in longitudinal course, motor alterations are common; hospitalizations are frequent and large, the prognosis is unfavorable and highly disabling, basically due to the cognitive damage. Generally, the response to classic antipsychotic treatment is irregular, requiring benzodiazepines and electroconvulsive therapy, which promote GABA-A receptor binding33mainly in frontoparietal circuits, showing alterations in that brain region during fMRI, associated to motility processing.34,35 The anterior cingulated gyrus is associated to catatonia because damage in this area generates akinetic mutism, which consists in a remarkable apathy with indifference to pain, hungry or thirst, lack of motor initiative, abnormal movements, echopraxia and echolalia;5 mimicking at least eight (8) DSM V catatonia’s diagnosis criteria, and more strikingly directed to the akinetic form. Anterior cingulated circuit starts in cingulated cortex (24 Broadmann area), sending afferences to the striatum (caudate, putamen and accumbens nuclei) passing through the internal globus pallidus, ending in the locus nigger. Many of those inputs are connected to the supplementary motor area and association cortex, in the parietal lobe. This circuit regulates the spatial processing of movement, which is required for the correct performance of motor activity, and lesions of these brain regions generate abnormalities in the cognitive component of movement control.36 The performance of patients with catatonic schizophrenia in the visual and perception object-space tests is deficient37 (table 3); additionally, the object alternation task and the Iowa gambling task (for evaluate decision making) showed abnormalities as well, associated with the reduction of cerebral blood flow in left frontoparietal surface during the brain Single Photon Emission Computed Tomography (SPECT) with Tc-99mECD;38 in contrast, patients with paranoid schizophrenia don’t show a remarkable deficit on those tasks.39
Table 3. Prefrontal circuits and main neuropsychological deficit.
4. Simple schizophrenia and Prefrontal dorsolateral circuit
Simple schizophrenia was introduced by Diem, describing a disease without bold presence of delirious and hallucinations and with progressive cognitive impairment,40 seventy years later, Kraepelin included it as subtype of schizophrenia. Those patients usually do not show long periods of psychotic symptoms; contrary, social isolation and cognitive impairment induce an almost sure disability during the 4th or 5th decade of life.41 Cognitive damage includes a progressive impairment of intellectual coefficient, working memory and executive functions, which are associated to bilateral frontotemporal hipoperfusion SPECT.42 This combination is not shared for others subtypes of schizophrenia.43 Atypical antipsychotics and antidepressants have shown partial efficacy, which suggest that serotoninergic system is implicated in this subtype of the disease.44 However, no treatment option available to date produces a relevant improvement.45,46
The dorsolateral prefontal circuit starts at the 9th and 10th Broadmann’s areas in the lateral surface of prefrontal cortex, projecting to dorsolateral head of the caudate nucleus, internal globus pallidus and sustantia nigra pars reticulate, ending it up in the ventroanterior and mediodorsal thalamic nucleus and finally returns to its cortical place.5 Patients with dorsolateral prefrontal syndrome show disexecutivity with disturbances in planning, monitoring and working memory, besides sustained attention deficit, learning disabilities, and rigid, perseverative and poor logical thinking with marked social isolation.26,36 Furthermore, those patients present apathy, defined as a quantitative reduction of voluntary initiatives, linked to an underestimation of environmental elements.47 Neurocognitive damage caused by simple schizophrenia match with dorsolateral prefrontal syndrome,48 showing both an inefficient performance during Wisconsin Test.26,42 Besides, patients with simple schizophrenia have diffuse cerebral atrophy mainly in dorsolateral prefrontal cortex in MRI, associated to a prominent social retraction,49 showing among all schizophrenia subtypes that with the highest degree of cerebral atrophy.50
5. Hebephrenic schizophrenia
Hebephrenia was originally described by Ewald Hecker in 1871, being the most deteriorate forms of the disease prevailing disorganized behavior, and distortion of language and ideas production.51 The disability during the 3rd or 4rd decade of life is almost certain, and loss of life quality is the most dramatic of all schizophrenia subtypes.52 Currently it is known that the presence of disexecutive syndrome predicts the tenacity of thinking disorganization as a main symptom, associated to semantic and language alterations;53 in addition, frontotemporal dementia shows behavioral and language alterations, indistinguishable from the hebrephrenic symptoms,54,55 which suggests an extensive frontal involvement in this subtype of schizophrenia. Nevertheless, it has not been possible to identify a constant pattern of cortical affectation in MRI, which is always diffuse,14associated to a blood flow reduction in the right frontal lobe during SPECT, especially in Broca’s area; this situation is not manifested in patients with others subtypes of the disease. Disorganized thinking in schizophrenia is associated with a reduction of fractional anisotropy in medial longitudinal fasciculus, which connects all the language regions,56 justifying the alterations in the verbal fluency57and semantic in the hebephrenic patient.58 This subtype also presents orbital, cingulated59 and dorsolateral circuits disfunction,60 indicating a frontal multicircuit deterioration, involving a dark prognosis, even when the most problematic symptoms have been controlled.61 "> Extensive frontal damage induces a kind of aphasia different to the classical Wernicke and Broca descriptions; showing a preserved motor domain but deficient use of prepositions and predictive functions of speech.62 Additionally, incoherent, uninhibited and confabulatory language63 are elements strongly linked to the speech of hebephrenics.58
6. Integration: From Frontal Cortex to Hippocampus
The schizophrenic brain has an inhibitory interneuron deficit in prefrontal cortex and hippocampus,64 without molecular findings of cell death, therefore it is believed that those interneurons never made the radial and tangential migration process for their final inclusion to the cortex,65events which normally occur the last trimester of gestation and in the early neonatal period. Therefore, a subject with schizophrenia is born with those abnormalities but becomes clinically evident at adolescent, time when prefrontal cortex fully matures and myelinates.66 "> All prefrontal cortex circuits connect with hippocampus through the anterior thalami nuclei; regulating their activity and producing gamma oscillations, associated with awareness, thinking, attention and executivity,67 functions clearly affected in schizophrenia,68,69 suggesting that this illness is a condition of prefronto-hippocampal desynchronization.
The hippocampus shows an association with schizophrenia for three principal reasons: 1) the lack of prefrontal interneurons linked with the disease, appears also in the hippocampus;64,70 2) gamma oscillations normally recorded in the frontal cortex are a direct consequence of the hippocampal physiology, and schizophrenic patients have abnormalities in those oscillation;68 and 3) there is an extensive prefrontal-hippocampal network, which characterizes schizophrenia such as a disease significantly different from frontal syndromes. Every prefrontal circuits described previously (vide supra), establishes synaptic connections with anterior thalamic nuclei and hippocampus, developing memory formation, necessary for carry out every component of executivity,67 and each prefrontal circuits commands one or some neuropsychological functions involved in schizophrenia. Prefrontal cortex receives synapses from other thalamic nuclei, including medial dorsal, medial anterior, ventral anterior and pulvinar nucleus,71,72 moreover, thalamic and hippocampal afferences arriving to prefrontal cortex show a different pattern of distribution according to cortical layers; e.g., CA1 is connected to layer 5 and 6 of neocortex,73 and the medial dorsal thalamic nucleus which represents the main afferences of prefrontal cortex, projects to layer 3.74,75 Besides, the prefrontal cortex regulates afferences that come from ascending reticular system which arrive to the first layer, putting in execution process related to attention and working memory, associated to high-frequency oscillations and persistent discharge, synchronized indirectly by hippocampus;76,77 contributing also to the formation and recovery of memory.78 The most studied role of the hippocampus related to memory, is the ability to codify and separate related events, necessary for the execution of episodic and autobiographic memory,79 involved in schizophrenic delusions formations.80 However, the hippocampus modulates also cognitive, sensitive processing and decision making, which are tightly related to the disease;81,82 and could justify the presence of auditory hallucinations in schizophrenic patients, which have been observed in different functional neuroimaging techniques such as simultaneously hippocampal activation.83,84 The clearest cognitive function association between the hippocampus and schizophrenic brains is the recovering of items previously learned.85 Also, the ability to classify new learned items and differentiate them from older ones is deficient, especially when are related,86 these characteristics have been associated to a volume reduction and hippocampal hyperactivation.87 The three prefrontal circuits related to schizophrenia subtypes show connections in different hippocampal regions; the orbitofrontal circuit sends afferences to the ventral region of CA1, the posterior region of CA1 sends efferences to cingulated girus88 and dorsal lateral cortex ends in the rhinal sulcus, parahippocampus and subiculum.71The CA1 has been the object of recent functional neuroimaging reports in schizophrenic patients,89 showing an association between blood flow increase in this region and the presence of positive symptoms,90 as well as its posterior portion was associated with working spatial memory.91,92 Additionally, rat models of hippocampal global dysfunction develop disorganization and are used such as an experimental model for hebephrenic schizophrenia.93 Is supposed that abnormal hippocampal glutamatergic transmission, generates a re-entry circuit in the prefrontal cortex inducing “cognitive mistakes”, which determine a psychotic pattern of association with abnormal memories.94 Nevertheless, scientific evidence which links schizophrenia subtypes with dysfunction in specific hippocampal regions is almost absent, and a lot of studies have been developed in rat and primates brains through tracer techniques, which are impossible to perform in humans.88
Recently was identify four (4) different patterns of neuropsychological damage in schizophrenic patients e.g., with global cognitive impairment, difficult in faces recognition, disexecutivity, and without cognitive impairment;95 which represents an additional sample of the existence of various neuropsychological subtypes of schizophrenia, and matches to the four mean clinical subtypes of the disease; furthermore, the two more prevalent groups of schizophrenics in clinical practice, schizophrenia with or without deficit, show different affectation pattern of verbal working memory.96 Clearly, is difficult to find a schizophrenic patient with a precise subgroup of symptoms and specific neurocognitive damage, just because in patients with frontal damage exist the involvement of more than one prefrontal circuit, being difficult to find someone with a isolated lesion in just one of those circuits,5,26then, we propose that clinical heterogeneity in schizophrenic patients could arise from the combination of more than one prefrontal circuit impairment. The definitive physiopathology approximation to the clinical psychiatry, more than insist in clinical subtypes; will be possible developing neuropsychological clusters, letting to psychiatrists to have objective evidence of the patterns of brain damage induced by the different endophenotypes of schizophrenia. Finally, is evident the need of include the neuropsychological deficit in the diagnostic criteria; elements that will explain better the differences in cognitive impairment, disability, treatment response and prognosis from one patient to other.
Authors thank Prof. Dr. Antonio Eblen-Zajjur, for critical Reading of the manuscript. Partially supported by Dirección de Investigación y Producción Intelectual, Facultad de Ciencias de la Salud, Universidad de Carabobo.
9. Conflict of interest
Author declares no conflicts of interest.
- Lehman A, Lieberman J, Dixon L, McGlashan T, Miller A, Perkins D, Kreyenbuhl J. Practice Guideline for the treatment of patients with Schizophrenia. Americam Psychiatric Association. Second Edition. 2004: 165-171.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), 4th edition. American Psychiatric Association, Washington D.C. 2000: 14-21.
- Tandon R, Gaebel W, Barch DM, Bustillo J, Gur R, Heckers S, Carpenter W. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013 150: 3-10.
- De Leeuw M, Bohlken M, Mandl R, Kahn R & Vink M. Reduced fronto–striatal white matter integrity in schizophrenia patients and unaffected siblings: a DTI study. NPJ Schizophr. 2015 15001: 1-6.
- Tekin S & Cummings J. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002 53: 647-654.
- Mueser K & McGurk S. Schizophrenia. Lancet. 2004 19: 2063-2072.
- Weiden P, Buckley P & Grody M. Understanding and Treating ‘‘First-Episode’’ Schizophrenia. Psychiatr Clin North Am. 2007 30: 481–510.
- Primavera D, Bandecchi C, Lepori T, Sanna L, Nicotra E & Carpiniello B Does duration of untreated psychosis predict very long term outcome of schizophrenicdisorders? Results of a retrospective study. Ann Gen Psychiatry. 2012 11: 21.
- ablensky A. The diagnostic concept of schizophrenia: its history, evolution, and future prospects. Dialogues Clin Neurosci. 2010 Sep; 12(3): 271–287.
- Zald D & Andreotti C. Neuropsychological assessment of the orbital and ventromedial prefrontal cortex. Neuropsychologia. 2010 48: 3377-3391.
- Berry K, Bucci S, Kinderman P, Emsley R, & Corcoran R. An investigation of attributional style, theory of mind and executive functioning in acute paranoia and remission. Psychiatr Res. 2015 226: 84-90.
- Waldö M. The frontotemporal dementias. Psychiatr Clin North Am. 2015 38:193-209
- Radulescu A, Rubin D, Strey H & Mujica-Parodi L. Power Spectrum Scale Invariance Identifies Prefrontal Dysregulation in Paranoid Schizophrenia. Hum Brain Mapp. 2012 33: 1582-1593
- Zhang T, Koutsouleris N, Meisenzahl E & Davatzikos C. Heterogeneity of Structural Brain Changes in Subtypes of Schizophrenia Revealed Using Magnetic Resonance Imaging Pattern Analysis. Schizophr Bull. 2014 41: 74-84
- Kubera K. M, Sambataro F, Vasic N, Wolf N, Frasch K, Hirjak D. Source-based morphometry of gray matter volume in patients with schizophrenia who have persistent auditory verbal hallucinations. Prog Neuropsychopharmacol Biol Psychiatry. 2014 50: 102–109.
- Cummings JL. Organic psychoses. Delusional disorders and secondary mania. Psychiatr Clin North Am. 1986 Jun;9(2):293-311.
- Elliott R, Dolan R, FrithC. Dissosiable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cereb Cortex. 2000 10: 308-317.
- Corlett PR, Taylor J, Wang X, Fletcher P & Krystal J. Toward a neurobiology of delusions. Prog Neurobiol. 2010 92: 345–369.
- Zalewski C, Johnson-Selfridge M, Ohriner S, Zarrella K, Seltzer J. A Review of Neuropsychological Differences Between Paranoid and Nonparanoid Schizophrenia Patients. Schizophr Bull. 1998 24: 127-145.
- Wilder K, Weinberger D, Goldberg T. Operant conditioning and the orbitofrontal cortex in schizophrenic patients: unexpected evidence for intact functioning. Schizophr Res. 1998 30:169-174.
- Laurenson C, Gorwood P, Orsat M, Lhuillier JP, Le Gall D, Richard-Devantoy S. Cognitive control and schizophrenia: The greatest reliability of the Stroop task. Psychiatr Res. 2015 30: 10-6.
- Waltz J, Gold J. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007 93: 296-303.
- Srivastava S, Bhatia M, Gautam P, Rathi A. Current understanding of organic delusional disorder. A recent update. Delhi Psychiatr J. 2014 17: 18-24.
- Shamay-Tsoory S, Aharon-Peretz J, Levkovitz Y. The neuroanatomical basis of affective mentalizing in schizophrenia: comparison of patients with schizophrenia and patients with localized prefrontal lesions. Schizophr Res. 2007 90: 274-283.
- Dodell-Feder D, Tully LM, Lincoln SH, Hooker CI. The neural basis of theory of mind and its relationship to social functioning and social anhedonia in individuals with schizophrenia. Neuroimage Clin. 2013 27: 154-163.
- Fuster JM. The cognit: a network model of cortical representation. Int J Psychophysiol. 2006 May;60(2):125-132.
- Sokolov A. Psychopathological features and delusion of exaggerated self-esteem in endogenous manic-delusion states. Zh Nevrol Psikhiatr Im S S Korsakova. 2012 112: 18-24.
- Falkai P, Rossner MJ, Schulze TG, Hasan A, Brzózka MM, Malchow B, Honer WG, Schmitt A. Kraepelin revisited: schizophrenia from degeneration to failed regeneration. Mol Psychiatry. 2015 Jun;20(6):671-676.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM V), 5th edition. American Psychiatric Association, Washington D.C. 2013.
- Van den Ameele S, Sabbe B, Morrens M. Characteristics of catatonia in schizophrenia and mood disorders. Tijdschr Psychiatr. 2015 57: 94-98.
- Vlasova O, Beveridge A. Karl Jaspers' phenomenology in the light of histological and X-ray metaphors. Hist Psychiatry. 2014 Mar;25(1): 103-111.
- Starkstein S, Goldar J, Hodgkiss A. Karl Ludwig Kahlbaum's concept of catatonia. Hist Psychiatry. 1995 6: 201-207.
- Northoff G, Steinke R, Czcervenka C, Krause R, Ulrich S, Danos P, Kropf D, Otto H, Bogerts B. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999 67:445-50.
- Northoff G, Braus DF, Sartorius A, Khoram-Sefat D, Russ M, Eckert J, Herrig M, Leschinger A, Bogerts B, Henn FA. Reduced activation and altered laterality in two neuroleptic-naive catatonic patients during a motor task in functional MRI. Psychol Med. 1999 29: 997-1002.
- Scheuerecker J, Ufer S, Käpernick M, Wiesmann M, Brückmann H, Kraft E, Meisenzahl E. Cerebral network deficits in post-acute catatonic schizophrenic patients measured by fMRI. J Psychiatr Res. 2009 43: 607-614.
- Bonelli, R, Cummings J. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007 9: 141-151.
- Northoff G, Nagel D, Danos P, Leschinger A, Lerche J, Bogerts B. Impairment in visual–spatial function in catatonia: a neuropsychological investigation. Schizophr Res. 1999 37: 133–147.
- Northoff G, Steinke R, Nagel D, Czerwenka C, Grosser O, Danos P, Bogerts B. Right lower prefronto-parietal cortical dysfunction in akinetic catatonia: a combined study of neuropsychology and regional cerebral blood flow. Psychol med. 2000 30: 583-596.
- Bark R, Dieckmann S, Bogerts B, Northoff G. Deficit in decision making in catatonic schizophrenia: an exploratory study. Psychiatr Res. 2005 134: 131-141.
- Black D, Boffeli T. Simple schizophrenia: past, present, and future. Am J Psychiatry. 1989 146(10):1267-1273.
- McGlashan T, Fenton W. Classical subtypes for schizophrenia: literature review for DSM-IV. Schizophr Bull. 1991 17: 609-632.
- Serra-Mestres J, Gregory C, Tandon S, Stansfield A, Kemp P, McKenna P. Simple Schizophrenia Revisited: A Clinical, Neuropsychological, and Neuroimaging Analysis of Nine Cases. Schizophr Bull. 2000 26: 479-493.
- Yürekli Y, Bodur Z, Gülseren L, Mete L. Comparison of regional cerebral blood flow in deficit and nondeficit schizophrenic patients. Turk Psikiyatri Derg. 2003 14: 255-262.
- Quednow B, Geyer M, Halberstad A. Serotonin and schizophrenia. In: Muller P & Jacobs B (Eds), Handbook of behavioral neurobiology of serotonin.2010: 585-620.
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr Bull. 2014 41: 892-899.
- Vernon J, Grudnikoff E, Seidman A, Frazier T, Vemulapalli M, Pareek P, Correll C. Antidepressants for cognitive impairment in schizophrenia systematic review and meta-analysis. Schizophr Res. 2014 159: 385-394.
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006 16: 916-928.
- Kirkpatrick B, Galderisi S. Deficit schizophrenia: an update. World Psychiatry. 2008 7: 143-147.
- Galderisi S, Bucci P, Mucci A, D'Amato AC, Conforti R, Maj M. Simple schizophrenia: a controlled MRI and clinical /neuropsychological study. Psychiatr Res. 1999 91: 175-184.
- Wheeler A, Wessa M, Szeszko P, Foussias G, Chakravarty M, Lerch J, Voineskos A. Further Neuroimaging Evidence for the Deficit Subtype of Schizophrenia: A Cortical Connectomics Analysis. JAMA Psychiatry 2015 72: 446-455.
- Hoche A. El proceso esquizofrénico. Forma Hebefrénica. Alcmeon, Revista Argentina de Clínica Neuropsiquiátrica, 2009 15: 168-181
- Zouari L, Thabet J, Elloumi Z, Elleuch M, Zouari N, Maâlej M. Quality of life in patients with schizophrenia: a study of 100 cases. Encephale. 2012 38: 111-117.
- Xu J, Hui C, Longenecker J, Lee E, Chang W, Chan S, Chen E. Executive function as predictors of persistent thought disorder in first-episode schizophrenia: a one-year follow-up study. Schizophr Res. 2014 159: 465-470.
- Lamote H, Tan K, Verhoeven W. Frontotemporal dementia in a young woman with apparent schizophrenia. Ned Tijdschr Geneeskd. 1998 142: 1962-1965.
- Grewal GS, Kanagasundram S, Jambunathan S. Schizophrenia or frontotemporal dementia in a young Chinese female: a purview of possible diagnoses. Turk Psikiyatri Derg. 2011 22: 266-268.
- Asami T, Saito Y, Whitford TJ, Makris N, Niznikiewicz M, McCarley R, Kubicki M. Abnormalities of middle longitudinal fascicle and disorganization in patients withschizophrenia. Schizophr Res. 2013 143: 253-259.
- Badcock J, Dragović M, Garrett C, Jablensky A. Action (verb) fluency in schizophrenia: getting a grip on odd speech. Schizophr Res. 2011 126: 138-143.
- Hella P, Niemi J, Hintikka J, Otsa L, Tirkkonen J, Koponen H. Disordered semantic activation in disorganized discourse in schizophrenia: a new pragma-linguistic tool for structure and meaning reconstruction. Int J Lang Commun Disord. 2013 48: 320-328.
- Nenadic I, Maitra R, Langbein K, Dietzek M, Lorenz C, Smesny S, Gaser C. Brain structure in schizophrenia vs. psychotic bipolar I disorder: A VBM study. Schizophr Res. 2015 165: 212-219.
- Yoon J, Minzenberg M, Ursu S, Ryan B, Wendelken C, Ragland J, Carter C. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry. 2008 165: 1006-1014.
- Ortiz B, Gadelha A, Higuchia C, Noto C, Medeiros D, Pitta J, Bressan R. Disorganized symptoms predicted worse functioning outcome in schizophrenia patients with established illness. Clin Schizophr Relat Psychoses. 2015 24: 1-18.
- Zaytseva Y, Chan R, Pöppel E, Heinz A. Luria revisited: cognitive research in schizophrenia, past implications and future challenges. Philos Ethics Humanit Med. 2015 27,10: 4.
- Hardy C, Buckley A, Downey L, Lehmann M, Zimmerer V, Varley R, Crutch S, Rohrer J, Warrington E, Warren J. The Language Profile of Behavioral Variant Frontotemporal Dementia. J Alzheimers Dis. 2015 10, 50: 359-371.
- Benes F, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001 25: 1-27.
- Uribe E, Wix R. Migracion Neuronal y esquizofrenia. Neurociencias en Colombia. 2010 17: 45-60.
- Woo T. Neurobiology of schizophrenia onset. Curr Top in Behav Neurosci. 2014 16: 267-295.
- Gordon J. Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol. 2011 21(3):486-491.
- Gonzalez-Burgos G, Cho R, Lewis D. Alterations in Cortical Network Oscillations and Parvalbumin Neurons in Schizophrenia. Biol Psychiatry. 2015 77: 1031-1040.
- Senkowski D, Gallinat J. Dysfunctional Prefrontal Gamma Band Oscillations Reflect Working Memory and Other Cognitive Deficits in Schizophrenia. Biol Psychiatry. 2015 15: 1010-1019.
- Zhang Z, Reynolds G. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in thehippocampus in schizophrenia. Schizophr Res. 2002 55: 1-10.
- Goldman-Rakic P, Selemon L, Schwartz M. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984 12: 719-743.
- Jang S, Yeo S. Thalamocortical connections between the mediodorsal nucleus of the thalamus and prefrontal cortex in the human brain: a diffusion tensor tractographic study. Yonsei Med J. 2014 55(3): 709-714.
- Jay T, Witter M. Distribution of hippocampal CA1 and subicularefferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolusvulgaris leucoagglutinin. J Comp Neurol. 1991 313: 574–586.
- Miyamoto Y, Jinnai K. The inhibitory input from the substantia nigra to the mediodorsal nucleus neurons projecting to the prefrontal cortex in the cat. Brain Res. 1994 649: 313–318.
- Ray J, Price J. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1993 337: 1–31.
- Lewis D, Hashimoto T, Volk D. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005 6: 312–324.
- Miller E, Cohen J. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001 24: 167–202.
- Achim A, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. Br J Psychiatry. 2005 187: 500-509.
- Eichenbaum H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. 2004 44: 109-120.
- Lisman J, Coyle J, Green R, Javitt D, Benes F, Heckers S, Grace A. Circuitbased framework for understanding neurotransmitter and risk gene interactions inschizophrenia. Trends Neurosci. 2008 31: 234-242.
- Bast T, Feldon J. Hippocampal modulation of sensorimotor processes. Prog Neurobiol. 2003 70: 319-345.
- Eyler L, Olsen R, Nayak G, Mirzakhanian H, Brown G, Jeste D. Brain response correlates of decisional capacity in schizophrenia: A preliminary FMRI study. J Neuropsychiatry Clin Neurosci. 2007 19:137-144.
- Silbersweig D, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995 378: 176-179.
- Dierks T, Linden DE, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999 22: 615-621.
- Heckers S, Rauch S, Goff D, Savage C, Schacter D, Fischman A, Alpert N. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature Neurosciences. 1998 1: 318-323.
- Ongur D, Cullen T, Wolf D, Rohan M, Barreira P, Zalesak M, Heckers S. The
- neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 2006 63: 356-365.
- Weiss A, Zalesak M, DeWitt I, Goff D, Kunkel L, Heckers S. Impaired hippocampal function during the detection of novel words in schizophrenia. Biol Psychiatry. 2004 1: 668-675.
- Li M, Long C, Yang L. Hippocampal prefrontal circuit and disrupted functional connectivity in psychiatric and neurodegenerative disorders. Biomed Res Int. 2015: 810548.
- Talati P, Rane S, Kose S, Blackford JU, Gore J, Donahue M, Heckers S. Increased hippocampal CA1 cerebral blood volume in schizophrenia. Neuroimage: Clin. 2014 22: 359-364.
- Schobel S, Lewandowski N, Corcoran C, Moore H, Brown T, Malaspina D, Small S. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009 66: 938-946.
- Lee I, Kesner R. Time-dependent relationship betweenthe dorsal hippocampus and the prefrontal cortex inspatial memory. J Neurosci. 2003 15: 1517-1523.
- Spellman T, Rigotti M, Ahmari S, Fusi S, Gogos J, Gordon J. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015 18: 309-314.
- Olypher A, Klement D, Fenton A. Cognitive disorganization in hippocampus: a physiological model of the disorganization in psychosis. J Neurosci. 2006 4: 158-168.
- Tamminga C, Stan A, Wagner A. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010 167: 1178-1193.
- Rangel A, Muñoz C, Ocampo M, Quintero C, Escobar M, Botero S, García J. Neurocognitive subtypes of schizophrenia. Actas Esp Psiquiatr. 2015 43: 80-90.
- Alden E, Cobia D, Reilly J, Smith M. Cluster analysis differentiates high and low community functioning in schizophrenia: Subgroups differ on working memory but not other neurocognitive domains. Schizophr Res. 2015 168: 273-278.
| Recibido: 10 de agosto de 2016 | Aceptado: 05 de mayo de 2016 |
Correspondencia: Dr. Ezequiel Uribe. El Trigal, Valencia 2001, C.P. 3798, Venezuela. Tel: +58-241-8666259, Fax: +58-241-8685321. E-mail: euribe@uc.edu.ve, ezequiel.uribe@hotmail.com
Este es un artículo de libre acceso distribuido bajo los términos de la licencia de Creative Commons, (http://creativecommons.org/licenses/by-nc/3.0), que permite el uso no comercial, distribución y reproducción en algún medio, siempre que la obra original sea debidamente citada.
