Preferencia condicionada hacia el mismo sexo y niveles de testosterona en ratas macho
- Inicio
- Comité Editorial
- Lineamientos
- Carta de Cesión de Derechos
- Información Legal
- Acerca de la Revista
- Bases de Datos
- Contacto
- ISSN 2007-3054
- Centro de Investigaciones Cerebrales
Universidad Veracruzana
Díaz-Estrada Víctor X1, Tecamachaltzi-Silvarán Miriam B2, Barradas-Moctezuma Miriam2, Herrera-Covarrubias Deissy3, Ismail Nafissa3, Coria-Avila Genaro A4*
1Licenciatura en Química Farmacéutica Biológica, Universidad Veracruzana, México. 2Doctorado en Investigaciones Cerebrales, Universidad Veracruzana, México. 3School of Psychology, University of Ottawa,. Canada. 4Centro de Investigaciones Cerebrales, Universidad Veracruzana,México.
Resumen/Abstract
Introducción
Material y métodos
Resultados
Discusión
Conclusiones
Agradecimientos
Referencias
Correspondencia
Introducción: En el pasado, diferentes estudios evaluaron los niveles de testosterona (T) en suero en hombres homosexuales, sugiriendo que la T no juega ningún papel en la expresión de la preferencia hacia el mismo sexo. Nuestro laboratorio ha demostrado que la preferencia de pareja del mismo sexo puede ser condicionada en ratas macho que cohabitan bajo los efectos de un agonista del receptor de tipo D2 como quinpirole (QNP). Objetivos: En el presente trabajo utilizamos ratas para explorar los niveles de T después de que una preferencia hacia el mismo sexo se ha condicionado (aprendida). Material y Métodos: Los machos recibieron solución salina o QNP y se pusieron en su caja solos (-) o en cohabitación (+) durante 24 h con otro macho como estímulo condicionado. Esto se repitió cada 4 días, por un total de tres ensayos. En una prueba final libre de drogas se evaluó la preferencia socio/sexual entre macho y hembra. Cuatro días después se evaluó los niveles séricos de T en tres momentos diferentes (0, 15, 30 min) después de la exposición al olor condicionado. Resultados: Sólo los machos del grupo QNP+ mostraron una preferencia socio/sexual por el macho. Los niveles de T estuvieron incrementados desde tiempo 0 en los grupos de Salina+ y QNP+, indicando que T se eleva por la convivencia social y no por la preferencia hacia el mismo sexo. Conclusiones: Estos resultados indican que la testosterona no controla preferencias de pareja hacia el mismo sexo, independientemente de su origen (innata o condicionada).
Palabras clave: Aprendizaje, Condicionamiento, Dopamina, Testosterona, Homosexual, Quinpirole.
Introduction: In the past, different studies assessed the levels of serum testosterone (T) in homosexual men and concluded that it plays no role in the expression of same-sex preference. We have shown that same-sex partner preference can be conditioned in male rats that cohabitate under the effects of a D2-type receptor agonist like quinpirole (QNP). Objective: Herein, we used rats to explore the levels of T after a conditioned same-sex preference. Methods: Males received saline or QNP and were left alone (-) in their home cage or allowed to cohabitate (+) during 24 h with a male as conditioned stimulus. This was repeated every 4 days, for a total of three trials. In a drug-free final test we assessed socio/sexual partner preference between male and female. Four days later we assessed serum levels of T at three different times (0, 15, 30 min) after exposure to the conditioned odor. Results: Only QNP+ males displayed a socio/sexual preference for the scented male over the sexually receptive female. The levels of T were increased since time 0 in the groups Saline+ and QNP+, indicating that it was cohabitation, and not the same-sex preference what caused the increase of T. Conclusion: These results indicate that testosterone does not mediate same-sex partner preferences regardless of its origin (innate or conditioned).
Key words: Learning,Cconditioning, Dopamine, Testosterone, Homosexual, Quinpirole.
In the present study we examined the serum levels of testosterone in male rats that developed a conditioned same-sex partner preference via Pavlovian associations. Classical or Pavlovian learning occurs when a neutral cue gains incentive value after being associated in contingency and contiguity with an unconditioned stimulus (UCS) that produces an unconditioned response (UCR). After some repetitions, the neutral cue functions as a predictor of the UCS and becomes a conditioned stimulus (CS) capable of inducing a conditioned response (CR). In our laboratory we have systematically used a stimulus male rat scented with almond odor as a neutral cue, which becomes conditioned and gains incentive value for an experimental male by the association with injections of the D2-type receptor agonist quinpirole (QNP).1-3
Previous studies from other laboratories have shown that a low dose of non-selective dopaminergic drugs (i.e. apomorphine) or a selective D2 agonist (QNP) facilitate the development of pair bonds in monogamous rodents without the need of mating.4-8 Such effect is observed after systemic treatment, and also after intracerebral injections in the nucleus accumbens. The activation of D2-type receptors can be considered as the UCS that would normally occur during sex.4,9; Consequently, cohabitation without mating under the influence of QNP facilitates an association with specific partner cues that gain incentive value after some repetitions. In our former studies males were treated systemically with QNP or saline, and then were either left alone in their home boxes or allowed to cohabitate with an almond-scented stimulus male for 24 h, every 4 days for a total of 3 conditioning trials. Four days after the last trial we carried out a drug-free test to assess the socio/sexual preference for a novel sexually-receptive female or for the stimulus male.1,3 Very consistently, we have found that those male rats treated with QNP and allowed to cohabitate with another male display more contacts, more visits, spend more time in close contact with him and display female-like proceptive behavior (i.e. solicitations). Such preference occurs even in the presence of a sexually receptive female, which is not preferred despite the fact that she represents a cluster of natural cues that function as powerful UCSs to trigger sexual motivation. In addition, conditioned males display more non-contact erections when they are presented to the stimulus male behind a wire mesh screen, and fewer when they are presented to the sexually-receptive female. This indicates that they are more sexually-aroused by the sensory cues from the male. By contrast, the other groups always prefer the receptive female and display more non-contact erections before her. Accordingly, a conditioned same-sex preference is formed during the conditioning trials by the association of the male cues (i.e. almond scent) and the UCR induced by QNP, but it is expressed in subsequent tests without the need of QNP, as a learned preference. Although human homosexuality is more complex and may include a broader behavioral repertoire we consider that more visits, contacts, time spent together, female-like solicitations and non-contact erections are sufficient evidence to consider this as a rodent model of learned same-sex preference. Having models that help us understand the role of Pavlovian conditioning on brain function and its effects on the development of learned partner preferences is of great relevance for the behavioral neurosciences.
In the past, several studies explored the possibility that same-sex partner preferences were mediated by alterations in the levels of gonadal hormones, like testosterone. For instance, some studies in the 70´s showed that testosterone levels were somewhat lower in homosexual men as compared to heterosexual controls.10-12 In those studies men were classified according to the Kinsey scale as primarily homosexuals (class 5) or exclusive homosexuals (class 6). Interestingly, the levels of testosterone were significantly lower in those men class 6, with no history of heterosexual intercourse in the past, as compared to homosexual men class 5, with some heterosexual experience. Nevertheless, those findings have not been systematically replicated since other studies have found no differences in testosterone between homosexual and heterosexual men.13 Furthermore, recent studies on rat models of same-sex partner preference (e.g. by disrupting estradiol prenatally) have found no alterations in the levels of testosterone in adulthood.14 Accordingly, there is a general agreement upon the idea the endocrine factors play no role in the expression of homosexual partner preferences.15 However, as far as we know there are no studies that explored the levels of testosterone in males that learned to displayed a same-sex partner preference. Indeed, the levels of testosterone can increase after exposure to an olfactory conditioned cue paired with copulation.16 Thus, in the present study, we hypothesized that serum levels of testosterone would increase in male rats after exposure to a conditioned olfactory cue that triggered learned same-sex partner preference.
2.1. Subjects
Thirty-eight Wistar (W) male rats were used as experimental subjects to be conditioned, and 10 W males and 10 W females were used as stimulus. All were purchased from a certified laboratory animal supplier (Rismartâ), and had similar body weights (250-300 g) at the start of this study. Stimulus rats were housed by sex in groups of 5 in Plexiglas cages with a thin layer of aspen chip (Rismart), whereas experimental rats were housed in individual cages (except during the conditioning trials when they were allowed to cohabitate). All rats were maintained at room temperature on a reverse 12:12 h light/dark cycle (lights off at 08:00 h), at the Centro de Investigaciones Cerebrales, Universidad Veracruzana, Mexico. Water and rodent feed (Rismart) were provided ad libitum. All the experimental procedures were carried out according to the Official Mexican Norm NOM-062-ZOO-1999 for use and care of laboratory animals.
2.2. Drugs
Some males were treated with the dopamine D2-type receptor agonist quinpirole dihydrochloride (QNP) (SigmaÒ; St. Louis, MO). It was dissolved in 0.9% physiological saline and was injected intraperitoneally (i.p) in a dose of 1.25 mg/kg [as in 1-3,8] in a volume of 1 ml/kg 1 min before every conditioning trial in which they cohabited with another male (QNP+) or were left alone in their home cage (QNP-). Other rats were injected i.p. with 1 ml/kg of physiological saline (injectable grade) 1 minute before cohabitation (Saline+), and another group was left intact (Intact-).
2.3. Partner conditioning
All experimental animals spent 10 days in single cages before conditioning. Then, every conditioning trial lasted 24 h (beginning at 12:00 h and finishing at 12:00 h of the following day), and occurred every 4 days, for a total of three trials. During conditioning, experimental rats received their treatment (as explained above in 2.2 Drugs) 1 min before being placed into a plexiglas cage (20cm x 30cm x 45cm) that either contained a stimulus male rat (groups QNP+ and Saline+) or was empty (groups Intact-, QNP-). The stimulus male was scented with 0.5 ml of almond extract (DeimanÒ Mexico), applied on the back and neck. Almond extract served as a CS to facilitate recognition during the partner preference test, and the same couple cohabitated during every conditioning trial.
2.4. Sexual training and surgery
As in our previous experiments,2,3,17 stimulus males had received several trials of multiejaculatory sexual experience with receptive females prior to the start of the experiment, whereas experimental males were sexually naïve. Stimulus females were ovariectomized (OVX) and primed fully with subcutaneous (sc) injections of estradiol benzoate (10 μg) 48 h and progesterone (500 μg) 4 h before each test. For ovariectomy, females were anesthetized with a mixture of ketamine hydrochloride (50 mg/ml) and xylazine hydrochloride (4 mg/ml), mixed at a ratio of 4:3, respectively, and was injected i.p. in a volume of 1 ml/kg of body weight. Anesthetized females were then OVX bilaterally via a lumbar incision. Post-surgical treatment included three days of sc injections of flunixin meglumine (2.5 mg/kg) for analgesia, and enrofloxacine (5 mg/kg) every 24 h to prevent post-surgical bacterial infections. All females were given a week of post-surgical recovery before they were included in a final partner preference test.
2.5. Partner preference test
Preference was tested as in our previous studies four days after the final conditioning trial and it was drug-free. During the preference test, experimental rats were placed into a three-compartment chamber that had a thin layer of aspen chip. The start compartment (20cm x 30cm x 45cm) was connected to the two goal compartments by a T-shaped transparent tunnel of 20 cm in length. One goal compartment (same size as the start compartment) contained the scented male, and the other goal compartment contained an unscented sexually receptive female. The two stimulus partners (male and female) wore rodent jackets, connected to an elastic 20 cm in length, which allowed them to roam within their own chamber, but not beyond. Thus, experimental males were allowed to interact freely with the two rats that served as stimulus for 20 minutes.
Preference tests were video recorded and scored using the computerized software BOP (behavioral observation program).18 During the preference test experimental males were able to enter the goal compartments with the scented male and unscented female for interaction. As in previous studies, social partner preference was inferred when a male displayed more visits, more contacts and spent more time in close contact with the stimulus partners.1-3 However, we also assessed sexual preference by measuring latency and frequency of sexual behaviors. For example, we scored both latency and frequency of genital investigations, mounts, intromissions and ejaculations that the males displayed during the test. We also scored what we have referred to as female-like solicitations3 observed as head-wise orientations to the stimulus partners followed by a runaway.19
2.6. Non-contact erections
We additionally assessed sexual arousal in every male by measuring the frequency of non-contact erections evoked by the presence of the scented male, and compared them with the frequency of non-contact erections evoked by the presence of a sexually receptive female (in two separate and counterbalanced tests). The also test lasted for 20 min, and occurred just immediately before the partner preference test (e.g. animals tested for non-contact erections at 12:00 hrs were tested for partner preference approximately at 12:20 hrs). The testing rooms were contiguous, and animals were just moved from one room to another right away. Half of the experimental rats from the groups were exposed to the scented male on the first day, and exposed to the receptive female the following day. The other half of experimental rats from both groups were exposed to a sexually receptive female on the first day, and exposed to the scented male the following day. Counterbalancing the groups ruled out the possibility of a preference for the first partner. The non-contact erections test of the second day occurred approximately at the same hour for each rat.
The non-contact erections test was also drug-free, and occurred in a chamber with two compartments divided by a wire mesh. In one compartment we placed the experimental male and in the other compartment was the stimulus partner (either male or receptive female). This allowed visual, olfactory and auditory stimulation, but prevented direct contact between the experimental rat and the stimulus partner. The chamber had a transparent floor and a mirror in a 45° angle which allowed us to observe and quantify non-contact erections.3,20 The test occurred in a separate room, away from all males and females, to prevent the males from detecting receptive females. In addition, the floor and walls of the chamber were cleaned with water and alcohol after each test to eliminate conspecific odors. We determined differences in the total frequency of erections between the groups during exposure to a male or a sexually receptive female.
2.7. Hormone assays
After the partner preference test all the males were returned to their single home cages. Four days later all the males were olfactory exposed to a cotton gauge sprayed with 0.5 ml of almond scent and then blood samples were collected from different animals at three times (0, 15 or 30 min after exposure to the conditioned odor). Accordingly, the results would indicate baseline levels not affected by any social interaction (time 0) or after several minutes of exposure to the CS (time 15 and 30). For the blood samples, rats were deeply anesthetized with sodium pentobarbital (35mg/kg) and 3 ml of blood were collected from every animal via cardiac puncture. Samples were placed in vacutainer tubes containing no anticoagulant, and incubated in upright position at room temperature for 30 min to allow clotting. Tubes were then centrifuged for 15 min at 1000-2000 r.p.m. The supernatant was aspirated at room temperature and serum was kept in 500 mL aliquots and frozen at -20ºC during few days until processing. We used an Enzyme-Linked Immuno Sorbent Assay (ELISA) and a commercial kit for testosterone (ALPCOÒ 11-TESHU-E01) and followed the instructions as indicated by the supplier. The assays were read in an IMARKÒ microplate reader with the softwaremicroplate manager from Bio-Rad.
2.8. Statistical analysis
For all the behavioral variables we used a two-way analysis of variance (ANOVA) followed by a Tukey´s posthoc test to determine main effects of groups (Intact-, Saline+, QNP+, QNP-) or partner (scented male vs. unscented sexually receptive females) or any interaction between group x partner. To assess the levels of testosterone we used a two-way ANOVA to determine the main effects of groups (Intact-, Saline+, QNP+, QNP-), time (0, 15, 13 min) or any interaction between group x time. We used GraphPad Prism version 6.00 for Mac, GraphPad Software, La Jolla California USA, www.graphpad.com
3.1. Social and sexual behaviors.
The table 1 shows the means ± standard errors (SEM) for all the behaviors assessed during the 20-min test. The ANOVA showed some significant differences. For instance, there was a main effect of partner (male vs. female) in the number of visits F(1,30) = 5.3, p<0.05, and a trend for interaction between partner and group F(3,30) = 2.7, p=0.06. For the total time spent visiting the stimulus animal there was a main effect of partner F(1,29) = 20.5, p<0.05 and group F(3,29) >= 4.3, p<0.05. For the contact frequency there was a main effect of partner F(1,29) = 5.4, p<0.05 and group F(3,29) = 17.3, p<0.05. For the first contact latency there was a main effect of partner F(1,29) = 5.2, p< 0.05 and group F(3,29) = 2.7, p=0.05. For the total amount of time spent in body contact there was a main effect of partner F(1,29) = 4.4, p<0.05 and group F(3,29) = 8.8, p<0.05, and an interaction between partner and group F(3,29) = 2.8, p=0.05, although the posthoc test failed to detect specific differences. For the genital investigation frequency there was a main effect of partner F(1,29) = 11.4, p<0.05 and group F(3,29) = 2.9, p<0.05 and interaction F(3,29) = 3.9, p<0.05. The posthoc analysis showed specific differences between male and female in the saline+ group. For the first genital investigation latency there was a main effect of partner F(1,29)=8.1, p<0.05 and an interaction F(3,29)=3.2, p<0.05. For the frequency of female-like proceptive behavior (solicitations) there was a main effect of partner F(1,29)=12.3, p<0.05, group F(3,29)=10.6, p<0.05 and an interaction F(3,29)=12.1, p<0.05. The posthoc analysis showed that males from the QNP+ group displayed more female-like solicitations. For the first female-like solicitations latency there was a main effect of partner F(1,29)=12.5, p<0.05, group F(3,29)=16, p<0.05 and an interaction F(3,29)=7.7, p<0.05. The posthoc analysis showed that males from the QNP+ group displayed a shorter latency towards the male partner. There was a main effect of partner for the intromission frequency F(1,29)=6.1, p<0.05, and first intromission latency F(1,29)=12.3, p<0.05.
Table 1. Behaviors displayed toward a familiar male or a sexually-receptive female during a final drug-free preference test in males.
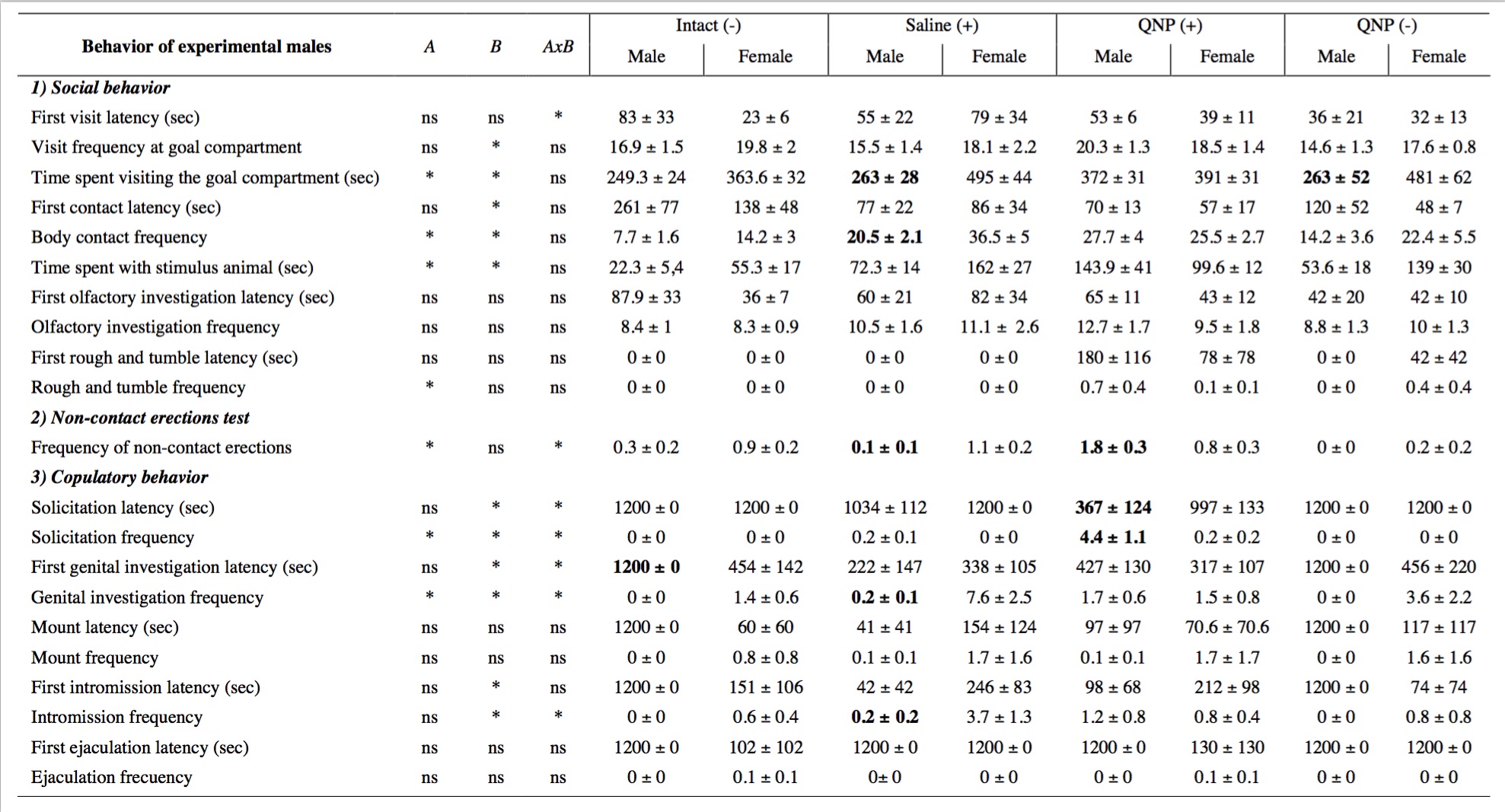
Previous to the preference test males underwent a conditioning process that consisted in treatment with quinpirole (QNP) or saline while cohabitating with another male (+) or alone (-). * indicates main effects for Group (A), Partner (B) or Interaction (AxB), ns=not significant. Bold numbers =p<0.05 within groups (male vs. female). Latencies are expressed in seconds.
3.2. Non-contact erections.
The ANOVA detected significant differences in the frequency of non-contact erections. There was a main effect of group F(3,29) = 5.7, p<0.05 and an interaction between group and partner F(3,29) = 7.7, p<0.05. The posthoc analysis showed that males from the Saline+ group displayed more non-contact erections when were exposed to a sexually receptive female, whereas the males from the QNP+ displayed more when were exposed to a male (Figure 1).
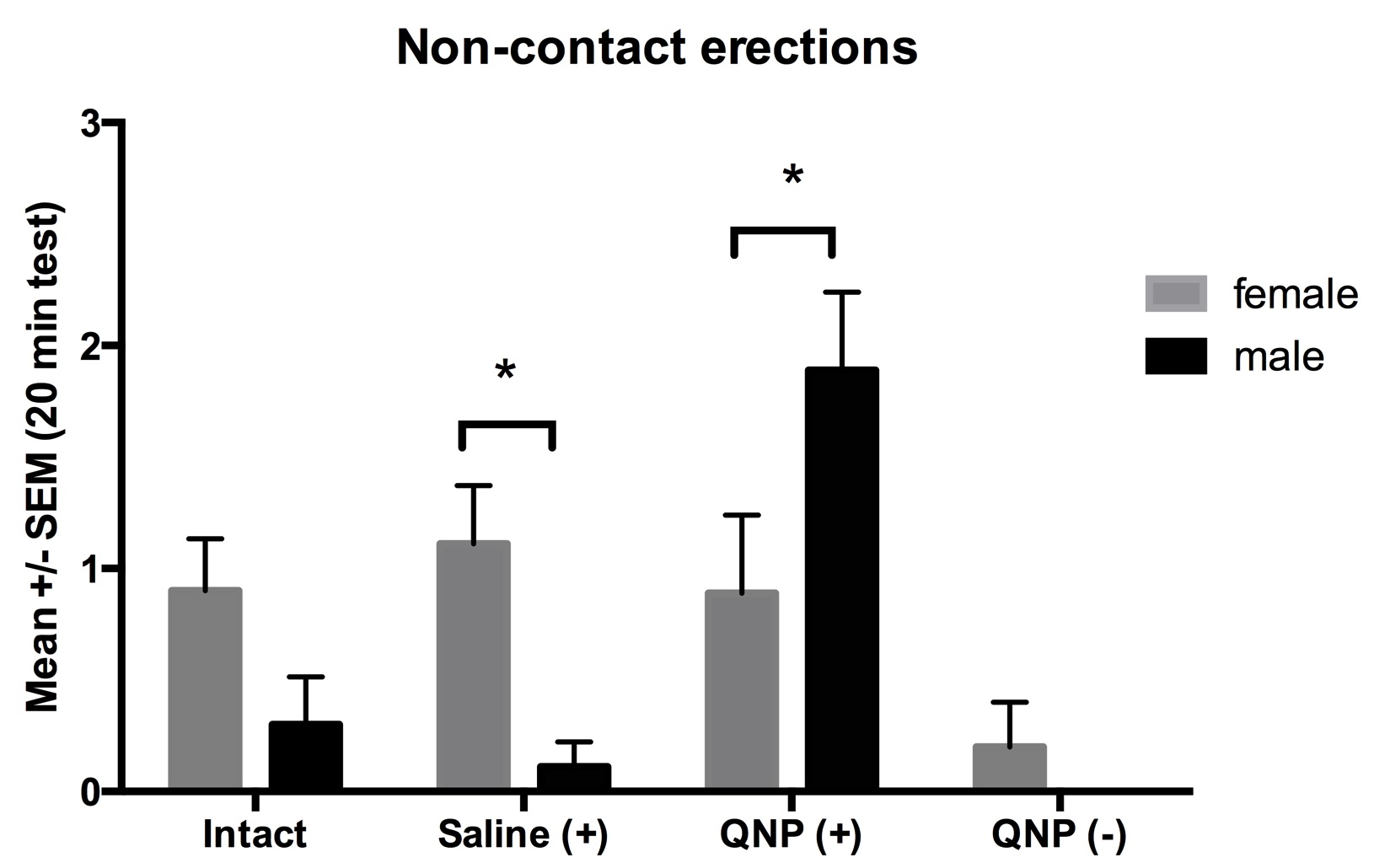
Figure 1. Mean ± SEM of non-contact erections displayed by experimental males during exposure to a male or a sexually-receptive female presented behind a wire mesh that allowed visual, olfactory and auditory contact between them. *=p<0.05 within groups.
3.3. Testosterone
The ANOVA detected a main effect of group F(3,26)=6.3, p<0.05 (Figure 2), but no main effect of time F(2,26)=1.6, p>0.05, or any interaction between group and time F(6,26)=0.5, p>0.05 (Figure 3). The posthoc test showed that males from the groups Saline+ and QNP+ expressed higher levels of serum testosterone as compared to the Intact- or QNP- group.
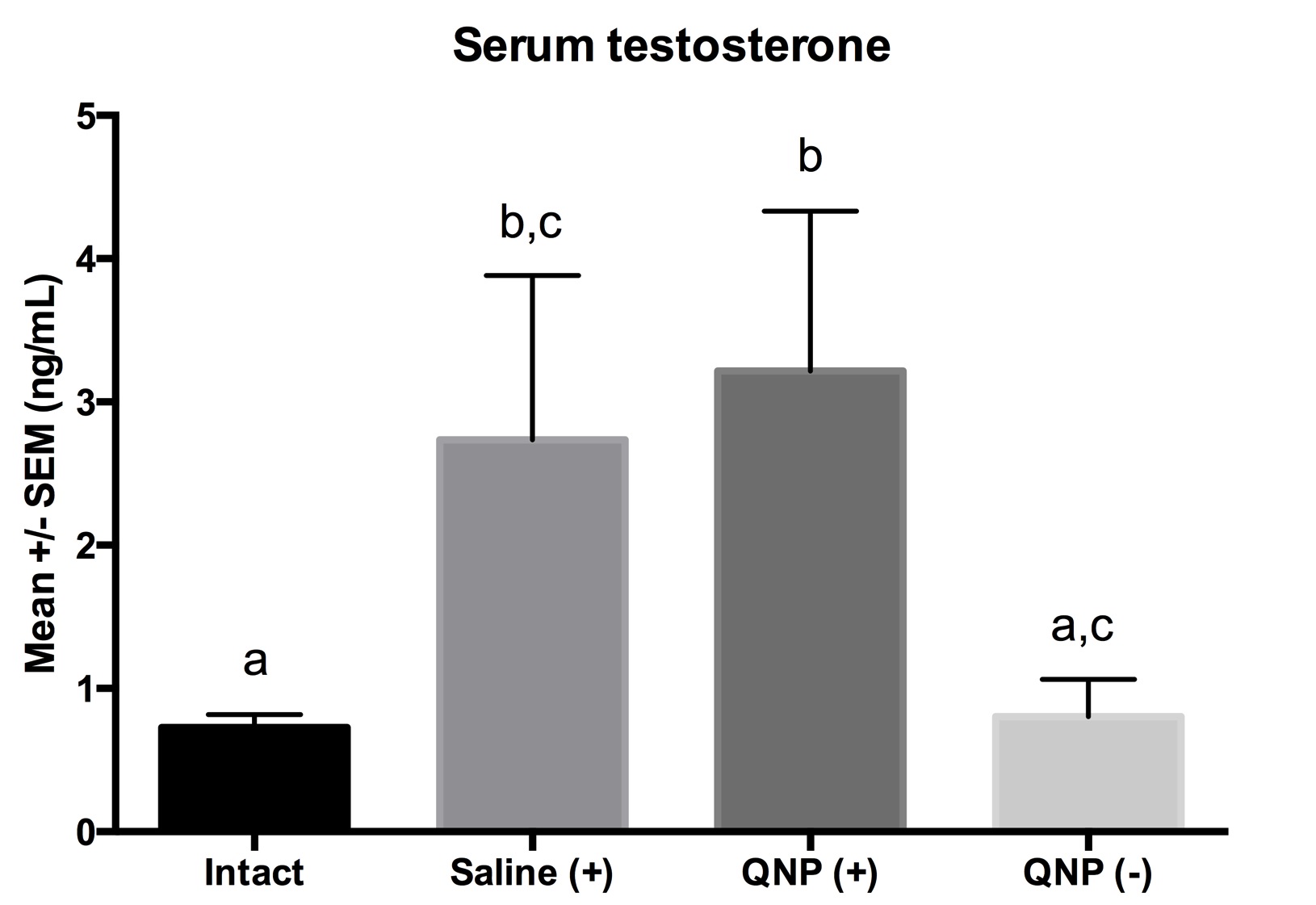
Figure 2. Mean ± SEM of serum levels of testosterone (ng/ml) in males with conditioned same-sex partner preference (QNP+) or controls (Intact-, Saline+, QNP-). Different letters = p<0.05.
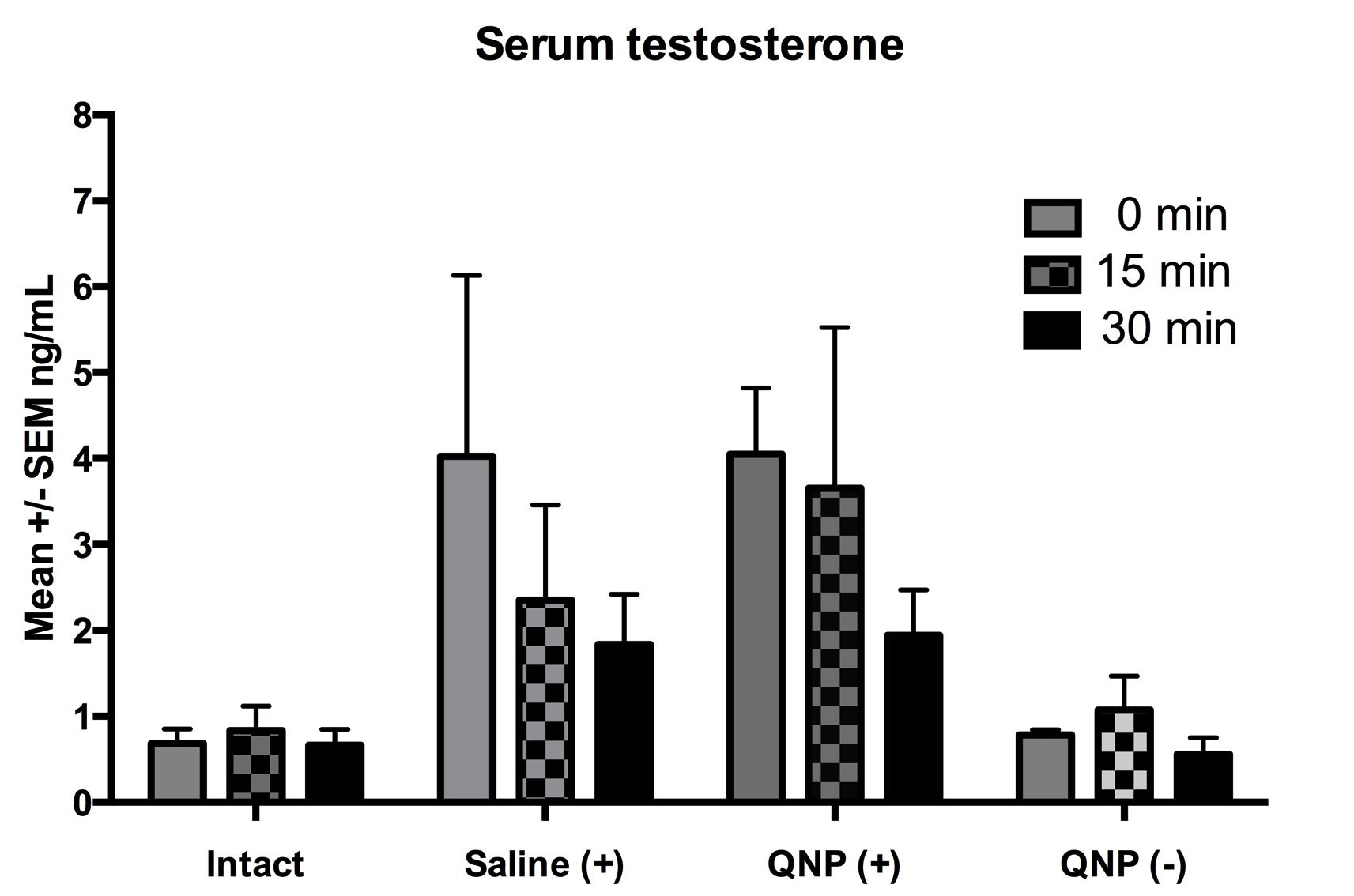
Figure 3. Mean ± SEM of serum levels of testosterone (ng/ml) at different times (0, 15, 30 min) after exposure to a conditioned odor (almond). In groups Saline+ and QNP+ the odor was paired with the male they cohabitated with. In groups Intact- and QNP- the odor was novel.
The results of the present study are in accordance with previous reports on the development of conditioned same-sex socio/sexual preference in male rats following a Pavlovian conditioning process.1-3 As expected, only males from the group QNP+ expressed a preference for the male partner over a sexually receptive female. The same-sex preference was mainly observed with more time in body contact (»60%), more female-like solicitations and more non-contact erections, and was not observed in the other groups (Intact-, QNP-, Saline+). In addition as compared to Saline+, those males in the QNP+ group expressed higher mean values (not statistically significant) for genital investigations toward the male, body contact frequency, and rough & tumble play. We have previously discussed that such results are not the pharmacological consequence of QNP alone. Peak plasma concentrations of QNP are observed about 15 min after administration, and up to 96% of the drug is recovered in the urine within the following 72 hrs.21 Our final preference test occurred 96 hrs after the last injection of QNP, therefore it is unlikely that the drug had an acute effect on those behaviors or on the non-contact erections. Accordingly, our results indicate that males from the QNP+ group were more sexually aroused by the presence of the male, and less aroused by the receptive female. Males from the QNP+ group expressed a rudimentary sexual desire for some kind of close interaction with the familiar stimulus male because they engage in female-like solicitations toward the male and display non-contact erections in the presence of the male (not observed in the other groups). However, as we have shown before males did not show hops, darts or lordosis, which are normally observed in a female rat that is proceptive and receptive. Furthermore, males did not mount the stimulus males nor attempted any other form of copulation with them. Thus, we cautiously have referred to this as a same-sex partner preference, with the nature of the preference being socio/sexual.1,3
4.1. Testosterone and same-sex preference
The aim of this study was to assess the levels of serum testosterone in male rats that learned a same-sex preference. Our results indicated the levels of testosterone were increased since time 0 only in those groups that cohabitated with another male (Saline+ and QNP+) but not in the two single-housed groups (Intact- and QNP-). Accordingly, the high testosterone levels were not related with same-sex partner preference (Figure 2). In addition, exposure to the odor during 15 or 30 min did not affect the testosterone levels either (Figure 3). Thus, learning a same-sex preference does not modify the levels of testosterone, but cohabitating with another male does. A former study16 showed that the serum levels of luteinizing hormone (LH) and testosterone increased in male rats exposed to the unconditioned odor from a sexually receptive female. Interestingly, a neutral odor (wintergreen) paired in contingency during 14 trials with copulation was powerful enough to induce in those males a conditioned response in the levels of LH and testosterone, which has been thought to facilitate the arousal observed in learned preferences. Accordingly, one possibility is that more trials in contingency would eventually induce an endocrine (testosterone) response in our males. Nevertheless, our results clearly indicate that changes in testosterone levels are not an explanation for the conditioned same-sex partner preference (Figure 3).
In the past, some human studies showed that homosexual men expressed lower levels of serum testosterone as compared to heterosexual controls.10-12 Interestingly, in the Pillard et al. study the authors further compared and found significant differences between primary homosexual men (7.91 ng/mL) and exclusively homosexual (6.12 ng/mL), although such differences did not account for gender identity, preferred sexual role, or frequency of orgasms. Nevertheless, those findings have not been systematically replicated in other groups of homosexual men13 nor in rats that display innate same-sex preference as a result of prenatal blockade of brain masculinization.14Accordingly, we can argue that regardless of the origin of same-sex preference (innate or conditioned) the levels of serum testosterone are not affected.
4.2. Testosterone and cohabitation
Our results show that the serum levels of testosterone were higher at time 0 in males that cohabitated (Saline+ and QNP+), as compared to those single-housed (Intact and QNP-). Time 0 represents baseline levels and not a conditioned response. In addition, we found no differences at time 15 or 30 after exposure to the conditioned odor. This indicates that it was due to cohabitation and not the partner preference what increased the levels of testosterone (Figures 2-3). Testosterone is not only part of the neuroendocrine cascade that facilitates sexual arousal, but also mediates social interaction in many species. For instance, it has been shown that the serum levels are increased in rats that cohabitate during four days (from 1.5 ng/ml baseline to »3.5 ng/ml), but are decreased after seven days of cohabitation when males have an overt establishment of behavioral dominance (2 ng/ml in dominant vs. 1 ng/ml subordinate).22 Therefore, the increased levels observed in groups Saline+ and QNP+ are likely the result of a hierarchy definition in progress between the stimulus and the experimental males.
4.3. Origen of conditioned same-sex preference
There is not a single neural system that controls the development of same-sex partner preferences. It has been discussed that a combination of areas involved in social recognition, motivation, reward, memory, and fear/anxiety mediate the formation, expression and maintenance of attachments and partner preferences.23 Accordingly, it is likely that the conditioning process that occurs between two males under the effects of QNP depends on a combination of enhanced recognition, motivation, reward and memory, and also on the inhibition of unconditioned fears or anxiety. Brain regions such as the amygdala and cortex may enhance recognition between the two males and disinhibit them to enhance motivation and decrease rejection responses.24,25Motivation and reward may mainly depend on the activity the nucleus accumbens (where QNP is believed to act, and crystalize the preference) but also on the systematic activity of medial amygdala, bed nucleus of the stria terminalis, lateral septum and medial preoptic area.26,27
Same-sex partner preference can be conditioned in male rats after intermittent cohabitation with another male, and under the effects of enhanced D2 activity. The serum levels of testosterone are not affected by the same-sex partner preference of the males. Altogether, these results indicate that testosterone does not mediate same-sex partner preferences regardless of its origin (innate or conditioned).
This research was supported by a basic science grant from SEP-CONACyT of Mexico (167773 to GACA), a graduate scholarship (353271 to MBTS).
7. Conflicto de intereses
Authors declare no conflict of interest.
- Cibrian-Llanderal T, Rosas-Aguilar V, Triana-Del Rio R, Perez CA, Manzo J, Garcia LI, Coria-Avila GA. Enhaced D2-type receptor activity facilitates the development of conditioned same-sex partner preference in male rats. Pharmacol Biochem Be 2012 102: 177-83.
- Triana-Del Rio R, Montero-Dominguez F, Cibrian-Llanderal T, Tecamachaltzi-Silvaran MB, Garcia LI, Manzo J, Hernandez ME, Coria-Avila GA. Same-sex cohabitation under the effects of quinpirole induces a conditioned socio-sexual partner preference in males, but not in female rats. Pharmacol Biochem Be 2011 99: 604-13.
- Triana-Del Rio R, Tecamachaltzi-Silvaran MB, Diaz-Estrada VX, Herrera-Covarrubias D, Corona-Morales AA, Pfaus JG, Coria-Avila GA. Conditioned same-sex partner preference in male rats is facilitated by oxytocin and dopamine: effect on sexually dimorphic brain nuclei. Behav Brain Res 2015 283: 69-77.
- Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci 2000 114: 173-83.
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci 2003 23: 3483-90.
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci 2006 9: 133-9.
- Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav 2004 83: 319-28.
- Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav Neurosci 1999 113: 602-11.
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res 1990 530: 345-8.
- Loraine JA, Ismail AA, Adamopoulos DA, Dove GA. Endocrine function in male and female homosexuals. Brit Med J 1970 4: 406-9.
- Kolodny RC, Masters WH, Hendryx J, Toro G. Plasma testosterone and semen analysis in male homosexuals. N Engl J Med 1971 285: 1170-4.
- Pillard RC, Rose RM, Sherwood M. Plasma testosterone levels in homosexual men. Arch Sex Behav 1974 3: 453-8.
- Barlow DH, Abel GG, Blanchard EB, Mavissakalian M. Plasma testosterone levels and male homosexuality: a failure to replicate. Arch Sex Behav 1974 3: 571-5.
- Olvera-Hernandez S, Chavira R, Fernandez-Guasti A. Prenatal letrozole produces a subpopulation of male rats with same-sex preference and arousal as well as female sexual behavior. Physiol Behav 2015 139: 403-11.
- Ryrie CG, Brown JC. Endocrine function in homosexuals. Brit Med J 1970 4: 685.
- Graham JM, Desjardins C. Classical conditioning: induction of luteinizing hormone and testosterone secretion in anticipation of sexual activity. Science 1980 210: 1039-41.
- Cibrian-Llanderal T, Triana-Del Rio R, Tecamachaltzi-Silvaran M, Pfaus JG, Manzo J, Garcia LI, Coria-Avila GA. Cohabitation between male rats after ejaculation: effects on conditioned partner preference. Physiol Behav 2014 128: 303-8.
- Cabilio S, Behavioral Observation Program. 1998, Concordia University: Montreal.
- Erskine MS. Solicitation behavior in the estrous female rat: a review. Horm Behav 1989 23: 473-502.
- Kelliher KR, Liu YC, Baum MJ, Sachs BD. Neuronal Fos activation in olfactory bulb and forebrain of male rats having erections in the presence of inaccessible estrous females. Neuroscience 1999 92: 1025-33.
- Whitaker NG, Lindstrom TD. Disposition and biotransformation of quinpirole, a new D-2 dopamine agonist antihypertensive agent, in mice, rats, dogs, and monkeys. Drug Metab Dispos 1987 15: 107-13.
- Hardy MP, et al. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod 2002 67: 1750-5.
- Coria-Avila GA, Manzo J, Garcia LI, Carrillo P, Miquel M, Pfaus JG. Neurobiology of social attachments. Neurosci Biobehav Rev 2014 43: 173-82.
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 2001 21: 8278-85.
- Keller M, Perrin G, Meurisse M, Ferreira G, Levy F. Cortical and medial amygdala are both involved in the formation of olfactory offspring memory in sheep. Eur J Neurosci 2004 20: 3433-41.
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci 2001 2: 129-36.
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci 2004 7: 1048-54.
| Recibido: 09 de octubre de 2015 | Aceptado: 12 de enero de 2016 |
Correspondencia: Genaro A. Coria-Avila Ph.D. Centro de Investigaciones Cerebrales, Universidad Veracruzana. Av. Luis Castelazo s/n Col. Industrial Ánimas. C.P. 91190. Xalapa, Veracruz, México. Phone: +52-228-8418900, Ext. 13609. Fax: +52-228-8418900 Ext. 13611. E-mail: gcoria@uv.mx
Este es un artículo de libre acceso distribuido bajo los términos de la licencia de Creative Commons, (http://creativecommons.org/licenses/by-nc/3.0), que permite el uso no comercial, distribución y reproducción en algún medio, siempre que la obra original sea debidamente citada.
