Effects of enriched environment on the expression of GABA receptors at the cerebellar vermis in the valproate rat model of autism
- Inicio
- Comité Editorial
- Lineamientos
- Carta de Cesión de Derechos
- Información Legal
- Acerca de la Revista
- Bases de Datos
- Contacto
- ISSN 2007-3054
- Centro de Investigaciones Cerebrales
Universidad Veracruzana
Pérez-Rodríguez Juan Antonioa, Toledo-Cárdenas María Rebecab, Herrera-Covarrubias Deissyb, Coria-Ávila Genaro Alfonsob, García-Hernández Luis Isaurob, Hernández-Aguilar María Elenab, Manzo-Denes Jorgeb
aDoctorado en Investigaciones Cerebrales, Universidad Veracruzana, Xalapa, Ver., México. bInstituto de Investigaciones Cerebrales, Universidad Veracruzana, Xalapa, Ver., México.
Abstract
Resumen
Introduction
Materials and Methods
Results
Discussion
Conclusions
Conflict of interest
Acknowledgments
References
Corresponding Author
Autism is a neurodevelopmental disorder in kids being the cerebellum a key affected area, and cerebellar alterations compromise the gamma-aminobutyric acid (GABA) neurotransmission which has stimulated efforts to develop treatments targeted to the GABA system. In addition, it is known that an enriched environment (EE) favorably affects behavior and perhaps neurotransmission. Thus, in the postnatal valproate model of autism in rats, we studied density modifications of GABAA and GABAB receptors at the cerebellar vermis by Western Blots analysis. Pup rats of both sexes were treated daily from postnatal days 6 to 12 with valproic acid (VPA). Control and experimental subjects of 30 and 90 days old were studied by sex, age, EE stimulation, and receptors. Results showed that GABAA and GABAB receptors in autistic rats were reduced depending on sex and age. The study at two ages showed that GABAA receptors in males decreased earlier than females with a long-lasting effect, while EE stimulation had a favorable impact showing that effectively prevents the reduction of GABAA receptors. In males, GABAB receptors were affected only at 30 days, suggesting that VPA produced a mild effect that was enough for these receptors to maintain by themselves in the long term, while these receptors were not affected at all in females. It is suggested that the higher sensitivity of male GABAA receptors to the VPA could mean that at least these receptors also play a role in the male-female tendency of autism and that EE is an appropriate preventive therapeutic approach.
Keywords: male autism; valproic acid; cerebellum; neurotransmission.
El autismo es un trastorno del neurodesarrollo que se presenta en niños y tiene al cerebelo como una de las principales áreas afectadas, incluyendo alteraciones en la neurotransmisión del ácido gamma-aminobutírico (GABA). Esto ha estimulado el desarrollo de tratamientos dirigidos al sistema GABAérgico, aunque también se sabe que el ambiente enriquecido (EE) estimula favorablemente la conducta y quizás la neurotransmisión. Por ello, utilizando en rata el modelo posnatal de autismo inducido con valproato, estudiamos la densidad de los receptores GABAA y GABAB en el vermis cerebelar por Western Blots. Crías de rata fueron tratadas diariamente del día posnatal 6 al 12 con ácido valproico (VPA), y se estudiaron sujetos controles y experimentales de 30 y 90 días de edad organizados por sexo, EE y receptores. Los resultados mostraron que los receptores GABAA y GABAB decrementaron dependiendo del sexo y edad, y se observó que con la edad los receptores GABAA de machos decrementaron antes que en las hembras y a largo plazo. La estimulación con el EE evitó la reducción de receptores GABAA. Los receptores GABAB no fueron afectados en las hembras, mientras que en los machos se afectaron sólo a los 30 días, sugiriendo que el VPA produjo un efecto leve que les permitió a los receptores mantenerse por sí mismos en el largo plazo. La alta sensibilidad al VPA de los receptores GABAA masculinos significa que al menos estos receptores juegan un papel en la prevalencia niño-niña del autismo y que el EE es un acercamiento terapeútico preventivo apropiado.
Palabras clave: autismo masculino; ácido valproico; cerebelo; neurotransmisión.
Autism is a neurodevelopmental disorder appearing in early infancy due to several anatomic and biochemical alterations in the central nervous system. The cerebellum is one of the crucial regions in the neurobiology of autism. This structure covers the dorsal part of the brain stem and contains about 50 billion neurons, almost half the total number of neurons in the brain.1 In autism, the cerebellum shows hypoplasia and a reduction in the number of Purkinje neurons. This appears to occur before the first year of life and may persist for several years.2 Furthermore, autism correlates with alterations of different circuits and neurotransmitters. In particular, animal models have shown that disruption of the gamma-aminobutyric acid (GABA) system plays a major role.3
GABA is involved in various circuits in the cerebellum. For example, Purkinje, Stellate, Golgi, and Basket neurons are GABAergic cells at the cerebellar cortex, while others are located in the deep dentate nucleus.4 Thus, GABA neurotransmission is central for the appropriate function of the cerebellum. In autism, the expression of the glutamic acid decarboxylase, a key enzyme in the anabolism of GABA from glutamate, is reduced in the cerebellum,5 a reduction that is also observed for GABA receptors.6,7 Accordingly, the disruption of cerebellar GABA neurotransmission may cause some behavioral alterations observed in autistic subjects, although it is unclear how deficient the neurotransmission should be to account for such impairments. However, this assumption has stimulated efforts to develop GABAergic pharmacological approaches to revert behaviors affected in autism.8-10 Interestingly, we have observed that non-pharmacological procedures, like those provided by an enriched environment (EE), could also help to revert autistic behaviors, perhaps by modulating different neurotransmission processes.
Several reports indicate that children with autism respond positively following stimulation by an EE. For instance, they show gains in IQ scores and improved language skills,11 enhance their role in home tasks,12 improved tactile sensations,13 and reduce the otherwise elevated heart rate and systolic pressure.14 Furthermore, in animal models of autism, evidence indicates that exposure to EE may revert behavioral alterations,15 enhance dendritic spine function,16 and increase cannabinoid receptors at the cerebellar cortex.17 Therefore, EE seems to be an appropriate approach to induce brain plasticity and enhance the function of neural circuits that may help modify aspects from molecules to behavior in autism. In addition, we have previously shown that EE has effects depending on sex, suggesting that from now on, research should involve a separate analysis of females and males.18 Thus, in the present study, we sought to explore the effects of sex, age, and EE on the expression of both ionotropic (GABAA) and metabotropic (GABAB) receptors at the cerebellar vermis in a rat model of autism.
Subjects
Pregnant Wistar female rats were obtained from the animal colony of our institute. They were maintained in a reversed light-dark cycle (12-12 hrs, light off at 0800 hrs), and each female was kept alone in a single cage with a continuous supply of food (rodent chow) and water. At birth, litters were always in their mother's cage. Four to six pups per litter, females and males, were randomly chosen and assigned to the experimental or control groups. The experimental group was the postnatal autistic model, i.e., from postnatal days 6 to 12, pups received a daily intraperitoneal injection of valproic acid (VPA) diluted in physiological saline solution at a dose of 150 mg/kg of body weight as described elsewhere.19 Control pups were injected just with the physiological saline solution in the same volume. Weaning occurred on day 21, then experimental or control animals were housed together until the end of experiments. All the protocols of the present study were approved by the local animal care committee (CICUAL, Reg. 2018-012) of the Institute for Brain Research at Universidad Veracruzana, Mexico, following the Official Mexican Norms (NOM-062-Z00-1999 and NOM-087-ECOL-SSA1-2003) and the Society for Neuroscience Policy on the use of Animals in Neuroscience Research.
Groups
Control and experimental subjects were separated by sex, then compared according to age (30 and 90 days old subjects, in order to compare young vs adults), type of environmental condition (Standard or EE stimulation), and type of receptor in the cerebellar vermis (GABAA and GABAB). The density of GABA receptors was analyzed by Western Blots (n=6 per group).
Standard and EE Stimulation
Animals were housed in acrylic cages throughout the study. However, starting ten days before reaching the respective age of its group, animals were placed daily for 2 hrs in a large arena (100 cm x 100 cm) between 1200 to 1600 hrs. The standard (Std) arena contained wood-chip exclusively as bedding. In contrast, the EE arena included structures and items to stimulate movement and senses with different surfaces, fruit odors, and jingle bell sounds.
Vermis collection
At the end of experimental procedures, animals were euthanized with an overdose of sodium pentobarbital (i.p. 120 mg/kg), according to the procedures approved by the local animal care committee. Then, the cerebellum was carefully extracted, and the vermis was isolated and stored in liquid nitrogen (-196oC) until assayed.
Western Blots
Protein concentration in the vermis tissue was determined using the Bradford method and expressed as arbitrary units. Protein samples (100 μg) were subjected to 7.5% SDS-PAGE under reducing conditions at 200 V (Mini-Protean III, Bio-Rad) and then transferred onto a nitrocellulose membrane at 100 V for 1:30 h. The nitrocellulose membrane 0.45 μm (162-0115, Bio-Rad) was blocked with TBS containing Tween-20 (0.1%) and milk (5%) for one hour. Next, the membrane was washed three times with a TBS-Tween-20 (0.1%) solution for 5 min each. The membrane was then incubated overnight at 4°C with the first antibodies [GABAA: Ra1-6 (E-8) sc-376282, 1:100; and GABAB: R1 (D-2) sc-166408, 1:50] from Santa Cruz Biotechnology. The following day, the membrane was washed three times and incubated with a secondary antibody (Goat anti-mouse IgG-AP, sc-2008, 1/1000, Santa Cruz Biotechnology) for one hour at room temperature. Bands were revealed using the AP Conjugate Substrate kit (170-6465, Bio-Rad), and densitometric analysis of the bands was performed using the Kodak image station 440-CF with the Kodak 1D 3.6 software.
Statistics
Data are summarized in graphs presented as bars showing means ± SEM. Control data were merged into a single bar because they did not show any difference. Statistics and graphs were performed using the Prism 9.3 software (GraphPad Software, USA). Data were analyzed by a one-way ANOVA followed by the Dunnett test to look for significant differences between control and VPA or EE stimulated groups. Significant differences were considered when p<0.05.
30 days old groups
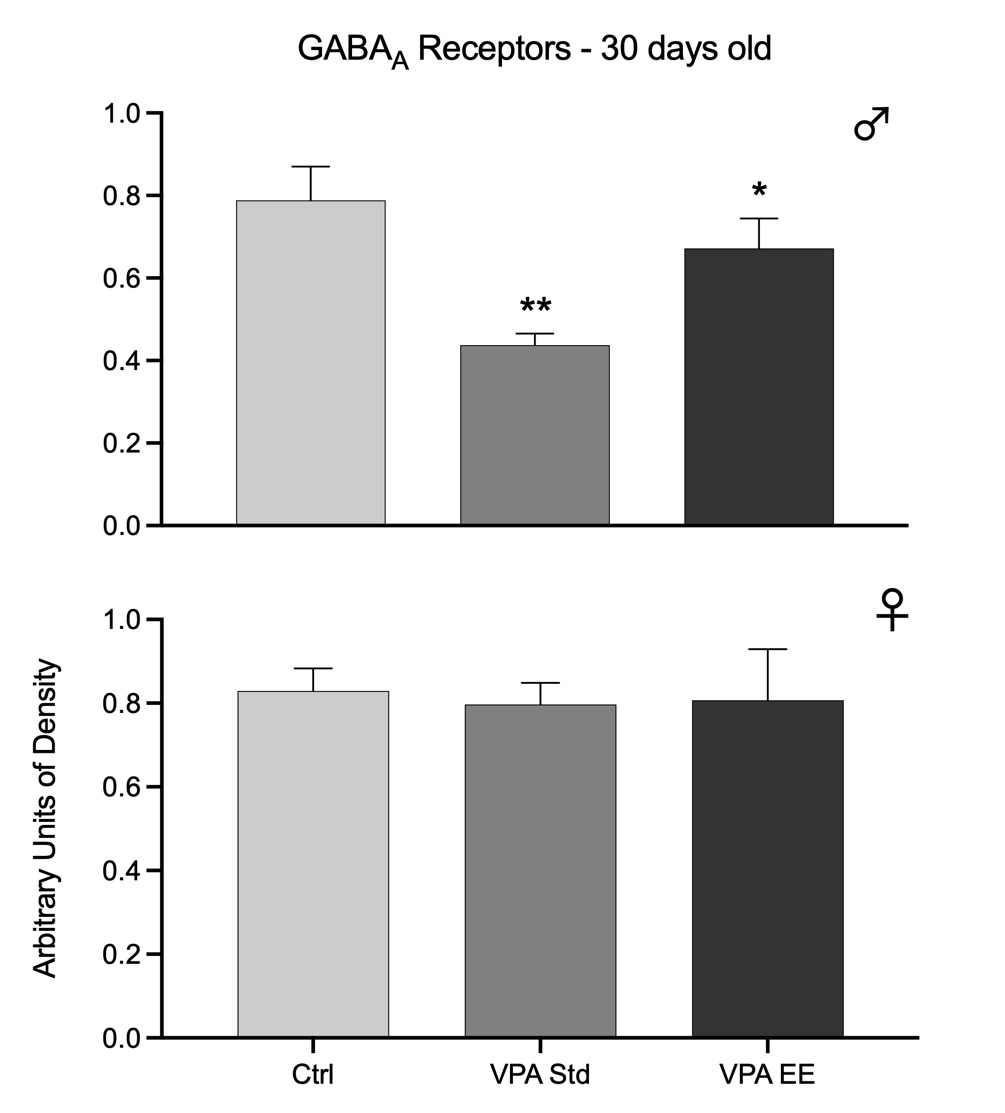
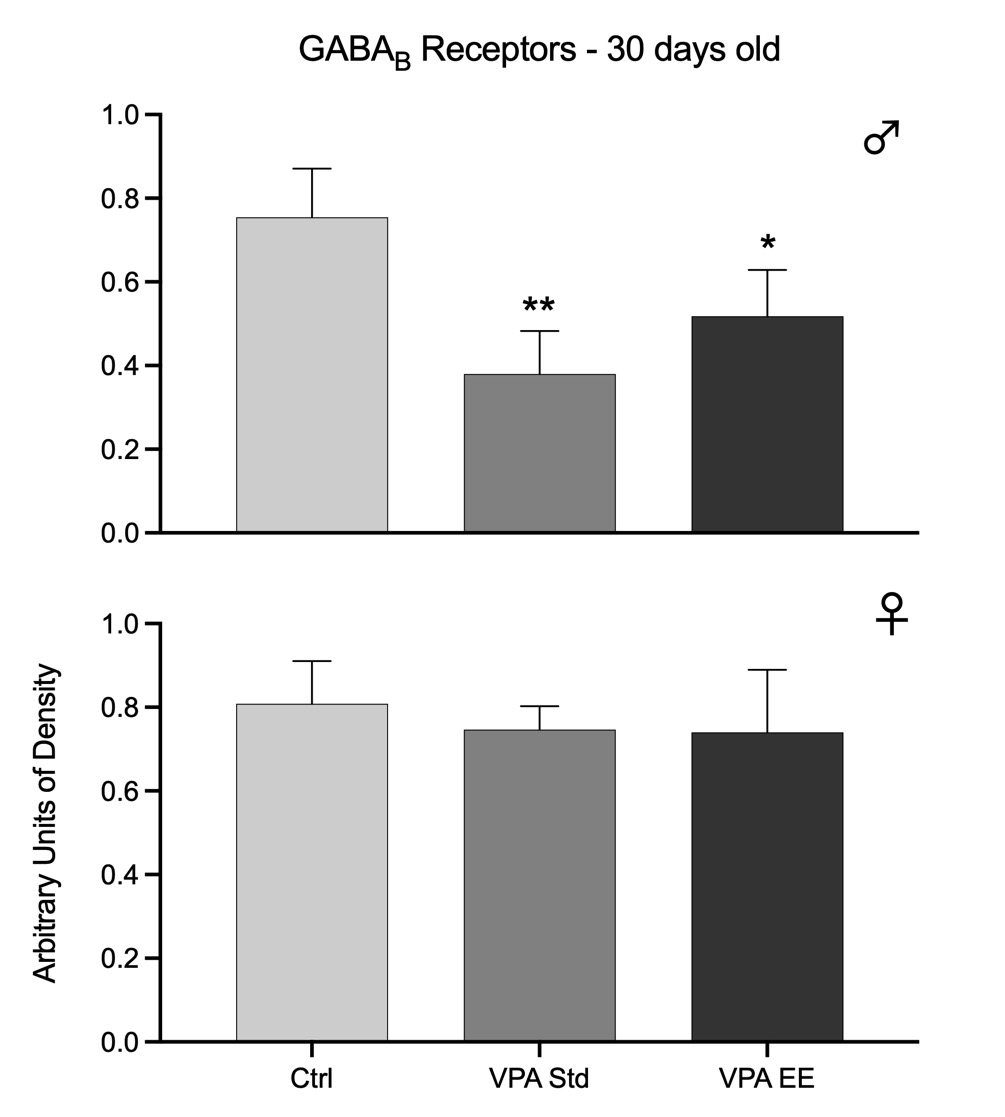
In the postnatal autistic VPA model, the density of GABAA and GABAB receptors was reduced only in males [GABAA: F(2,21) = 49.59, p<0.01; GABAB: F(2,21) = 24.53, p<0.01], while females showed no significant changes. Post hoc analyses showed that only VPA-treated males in Std conditions had an extreme decline in the density of receptors; such decline was prevented by EE stimulation, although the effect was not enough for the receptors to stay at the level of Ctrl values. In females, EE stimulation had no effect (Figs. 1 and 2).
Fig. 1. Western blots arbitrary units of the density of GABAA receptors at the cerebellar vermis of 30 days old male (upper graph) and female (lower graph) rats. Bars represent the control group (Ctrl), the VPA autistic group living in standard conditions (VPA Std), and the VPA autistic group stimulated with an enriched environment (VPA EE). Significant differences represent the comparison versus the Ctrl group. *p<0.05, **p<0.01.
Fig. 2. Western blots arbitrary units of the density of GABAB receptors at the cerebellar vermis of 30 days old male (upper graph) and female (lower graph) rats. Bars represent the control group (Ctrl), the VPA autistic group living in standard conditions (VPA Std), and the VPA autistic group stimulated with an enriched environment (VPA EE). Significant differences represent the comparison versus the Ctrl group. *p<0.05, **p<0.01.
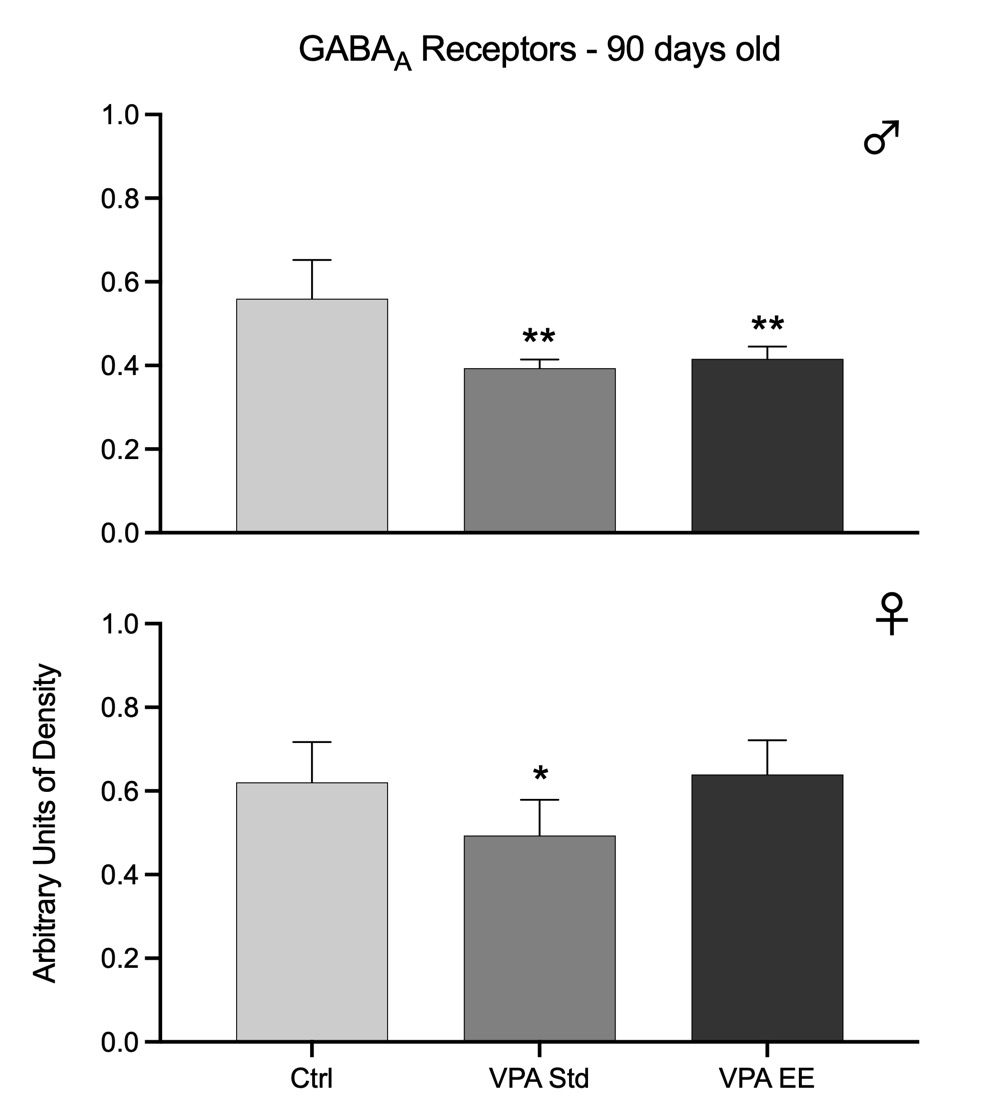
Fig. 3. Western blots arbitrary units of the density of GABAA receptors at the cerebellar vermis of 90 days old male (upper graph) and female (lower graph) rats. Bars represent the control group (Ctrl), the VPA autistic group living in standard conditions (VPA Std), and the VPA autistic group stimulated with an enriched environment (VPA EE). Significant differences represent the comparison versus the Ctrl group. *p<0.05, **p<0.01.
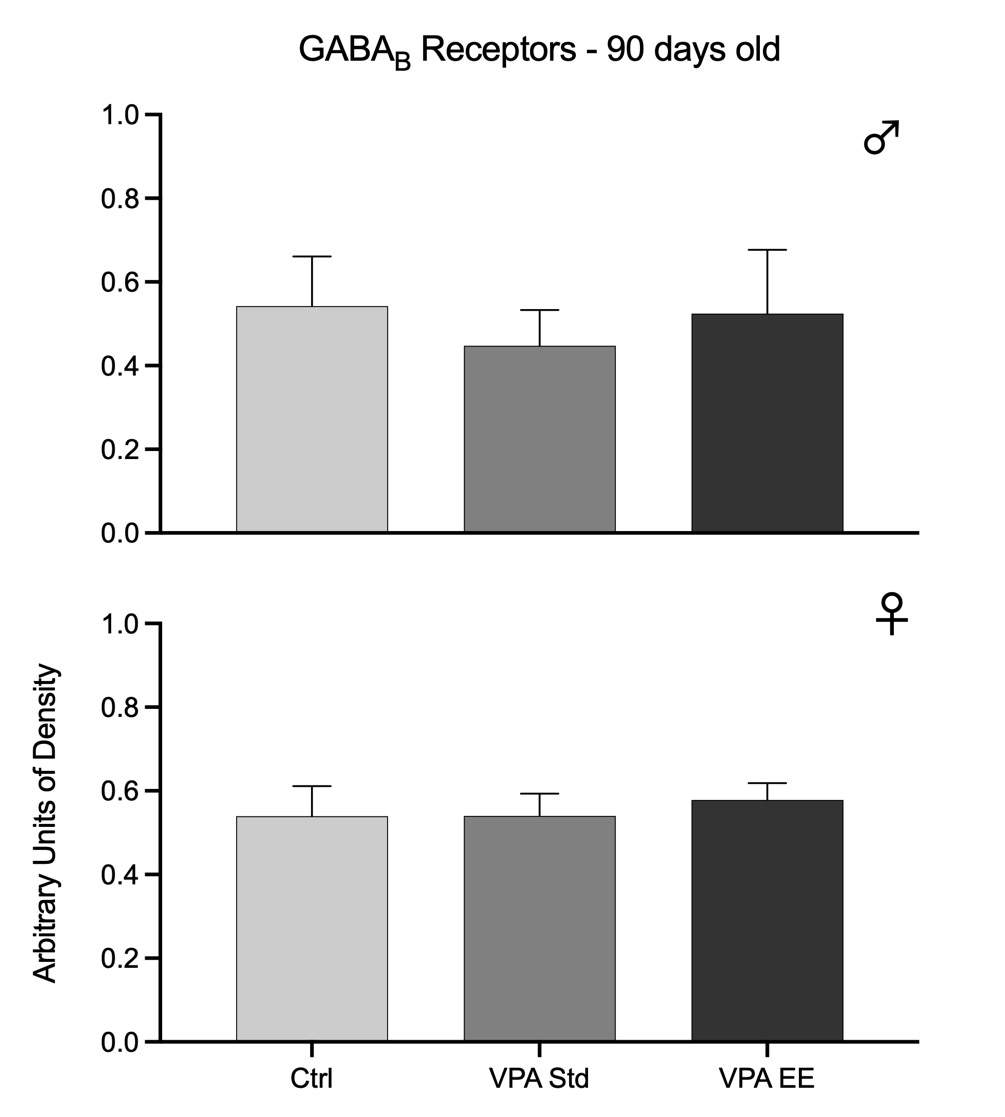
Fig. 4. Western blots arbitrary units of the density of GABAB receptors at the cerebellar vermis of 90 days old male (upper graph) and female (lower graph) rats. Bars represent the control group (Ctrl), the VPA autistic group living in standard conditions (VPA Std), and the VPA autistic group stimulated with an enriched environment (VPA EE). These data showed no significant differences.
90 days old groups
The density of GABAA receptors was significantly reduced in both males [F(2,21) = 15.31, p<0.01] and females [F(2,21) = 5.01, p<0.05]. Post hoc analyses showed that EE stimulation had no effect in males but in females successfully prevented the reduction in the density of receptors (Fig. 3). The density of GABAB receptors was not modified at this age in any sex, and EE stimulation had no effect (Fig. 4).
Longitudinal analysis
Data from both age groups show that GABAA receptors were affected in both sexes of the autistic model, but males were affected earlier than females and with higher intensity. Furthermore, data showed that males were responsive to EE stimulation at 30 days old but not at 90 days old, while females were responsive at 90 days old. GABAB receptors of the autistic model were affected in males at 30 days old and were partially responsive to EE stimulation. However, it was noteworthy that males showed no significant changes at 90 days old. These receptors were not affected at all in females.
GABA is a crucial neurotransmitter underlying the function of the cerebellum. Thus, the present study was designed to assess the expression of GABA receptors at the cerebellar vermis of rats in a valproate-induced model of autism. In addition, the present study analyzed the effects of sex, age, and enriched environment. GABAA and GABAB receptors showed different responses in our model of autistic rats, which depended on sex and age. The observed reduction of both types of receptors in 30-days old males agrees with the decrease of GABA receptors reported for several other brain areas in autistic humans, including the cerebellum.6,7 As the neurotransmitter itself and its GAD enzyme are also altered in autism,5 the whole GABA system is at the core of molecular changes of the disorder, suggesting that treatments that aim to target this system could become an appropriate pharmacological approach.20
It is noteworthy that reduction of GABA receptors in 30 days old subjects was observed only in males, a finding equivalent to that observed in humans;21 thus, it could underlie the higher masculine prevalence of autism. It is known that GABAA receptors are expressed in low density in the rat cerebellum at birth but show a linear increase each week until adulthood. In contrast, GABAB receptors already show a peak density at birth that remains steady until adulthood.22 Hence, it seems that both kinds of receptors were already active when VPA was administered in our postnatal model of autism. Thus, instead of a direct effect of VPA on receptor density, the outcome we obtained seems to be a sex-related mechanism in the molecular cascade that triggers autistic features. It is known that the density of at least GABAA receptors is modulated by its ligand occupancy,23 that VPA decreases the expression of androgen receptors in the cerebellum,24 that testosterone could account for the male prevalence of autism,25 that many neurosteroids account for the modulation of GABAA receptors,26 and that testosterone action affects protein synthesis.27 All this information suggests that some still unknown molecular interactions of VPA with male neurosteroids could be involved in the observed reduction of GABA receptors, resulting in autistic behaviors.
Stimulation of 30 day old rats with an EE prevented a decline in the density of both kinds of GABA receptors in the male cerebellum, although values did not stay at control levels. Notwithstanding, the finding is meaningful since it suggests that EE is appropriate for protecting molecular alterations triggered in autism. However, further research is needed to determine the precise paradigm for EE stimulation to keep receptors levels up to control values. Furthermore, it is essential to notice that the lack of effect in females indicates that EE stimulation will not modify receptors density above typical values. Our findings link to the literature showing different kinds of improvements following EE in the behavior of rats,15 dendritic spines in the hippocampus of mice,16 or behavior and cognitive abilities in children.11-14,18 Thus, it is suggested that an EE, in addition to the impact on GABA receptors, stimulates different neurobiological levels that could prevent behavioral alterations in autism. Some studies have found that acupuncture sensory stimulation in specific areas modulates the expression of GABA receptors,28,29 a topic that deserves further research to understand what EE does in the sensory system to impact GABA receptors in the cerebellum of autistic rats.
Data from 90 days old autistic rats add further significant information. Females and males showed decreased GABAA receptors at this age but no effect at all for GABAB receptors. EE stimulation was unable to produce any effect for GABAA receptors in males, while in females, it helped keep receptor levels up to control values; moreover, EE stimulation had no effect on GABAB receptors. Thus, again for this age, EE is a good approach according to female data, but the exact stimulation procedure for males deserves further studies. The differences observed between 30 and 90 days old rats could depend on the maturation of the subunits that organize GABA receptors. It is known that different subunits organize GABA receptors; GABAA receptors are formed by 19 subunits,30 and GABAB receptors by 2 subunits.31 Furthermore, for GABAA receptors, the subunits are different in the neonatal and adult brain,32 while the role of GABAB receptors on development is in the first weeks of life.33 Thus, as autism is a neurodevelopmental disorder, it is suggested that the different timeline of maturation of GABA receptors subunits, mainly those of GABAA, is underlying the results in our postnatal VPA model of autism and EE effectiveness.
The study at two ages gave a particular view of how both GABA receptors responded in a period by using the VPA postnatal autistic model. GABAA receptors decreased in 30 days old males and maintained this reduction for up to 90 days. Thus, GABAA receptors were affected early for males with a long-lasting effect. However, females showed a different picture; at the age of 30 days, GABAA receptors did not show any change, but they decreased at the age of 90 days. This result indicates that GABAA receptors in females are also sensitive to the VPA treatment, but they were affected late in time. On the other hand, the fact that EE had a positive effect on male GABAA receptors at 30 days but not at 90 days may suggest that a different stimulation paradigm is needed to produce a better outcome. Such inference came from female data where EE had a positive effect at the age of 90 days, suggesting that EE effectively maintains these receptors at this age and that the VPA effect on female receptors was not as severe as that observed in males. In correspondence, females showed no effect on GABAB receptors, while males were affected only at the age of 30 days, an effect not observed at the age of 90 days even without EE stimulation. Thus, it seems that VPA produced a mild impact on GABAB receptors that was enough for the receptors to maintain by themselves, perhaps by a neuronal plasticity mechanism.
What else does this information mean? Autism has a marked sex difference in humans, 3 out of 4 subjects with autism are males. Although the reason for this imbalance is widely unknown, it is theorized that it depends partially on male neurosteroids,25 together with the disruption of GABA circuits that accounts for autism appearance.34 Thus, our data also suggest that the high sensitivity of male GABAA receptors to the VPA postnatal model means that these receptors also play a role in the male-female tendency of autism.
GABA is a central neurotransmitter at the core of molecular changes occurring in autism. Here we observed that VPA, in the postnatal model of autism in rats, seems to affect some sex-related mechanisms in the molecular cascade that triggers autistic features, one of them producing a reduction of GABA receptors at the cerebellar vermis. Notwithstanding, EE helped in keeping up the levels of GABAA and GABAB receptors depending on sex and age. It is inferred that this approach is appropriate to prevent molecular changes from appearing in the autistic cerebellum.
The authors declare no conflicts of interests.
This work was supported by COVEICYDET, Veracruz State, Mexico [Grant 09 1238/2021 to JM]; and by CONACYT, Mexico [Scholarship 575611to JAPR].
1. Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci 2006 7(7): 511-522.
2. Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol 1997 7(2): 269-278.
3. Hampson DR, Blatt GJ. Autism spectrum disorders and neuropathology of the cerebellum. Front Neurosci-switz 2015 9: 420.
4. Schiffmann SN, Vanderhaeghen JJ. Immunocytochemical detection of GABAergic nerve cells in the human striatum and cerebellum using a gamma-aminobutyric acid antiserum. Neurochem Int 1990 17(1): 101-106.
5. Blatt GJ, Soghomonian JJ, Yip J. Glutamic acid decarboxylase (GAD) as a biomarker of GABAergic activity in autism: Impact on cerebellar circuitry and function. En: Blatt GJ, The neurochemical basis of autism. Springer Science 2010 95-111.
6. Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD. Expression of GABAb receptors is altered in brains of subjects with autism. Cerebellum 2009 8(1): 64-69.
7. Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABAa receptor downregulation in brains of subjects with autism. J Autism Dev Disord 2008 39(2): 223.
8. El-Ansary A. GABA and glutamate imbalance in autism and their reversal as novel hypothesis for effective treatment strategy. Autism Dev Disord 2020 18(3): 46-63.
9. Dai Y, Zhang L, Yu J, Zhou X, Ji Y, Wang K, Du X, Liu X, Tang Y, Deng S, Langley C, Li W, Zhang J, Feng J, Sahakian BJ, Luo Q, Li F. Improved symptoms following bumetanide treatment in children aged 3 to 6 years with autism spectrum disorder via GABAergic mechanisms: a randomized, double-blind, placebo-controlled trial. Medrxiv 2020: 2020.09.18.20197640.
10. Hadjikhani N, Zürcher NR, Rogier O, Ruest T, Hippolyte L, Ben-Ari Y, Lemonnier E. Improving emotional face perception in autism with diuretic bumetanide: A proof-of-concept behavioral and functional brain imaging pilot study. Autism 2015 19(2): 149-157.
11. Woo CC, Donnelly JH, Steinberg-Epstein R, Leon M. Environmental enrichment as a therapy for autism: A clinical trial replication and extension. Behav Neurosci 2015 129(4): 412-422.
12. Sood D, Szymanski M, Schranz C. Enriched home environment program for preschool children with autism spectrum disorders. J Occup Ther Sch Early Intervention 2015 8(1): 40-55.
13. Fernández-Lechuga AI, Nuñez-Arcos LY, Carrillo P, Garcia LI, Coria-Ávila GA, Toledo R, Hernández ME, Manzo J. Reduction of cutaneous von Frey thresholds in boys with autism following a year of tactile and emotional stimulation. Rev Mex Neurociencia 2021 22(3): 1-4.
14. Nuñez-Arcos LY, Fernández-Lechuga AI, Coria-Ávila GA, García LI, Toledo-Cárdenas MR, Hernández-Aguilar ME, Manzo J. Mozart para el autismo: Cuantificación de parámetros cardiorrespiratorios durante la escucha. eNeurobiol 2020 28(11): 131220.
15. Schneider T, Turczak J, Przewłocki R. Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: Issues for a therapeutic approach in autism. Neuropsychopharmacol 2006 31(1): 36-46.
16. Yamaguchi H, Hara Y, Ago Y, Takano E, Hasebe S, Nakazawa T, Hashimoto H, Matsuda T, Takuma K. Environmental enrichment attenuates behavioral abnormalities in valproic acid-exposed autism model mice. Behav Brain Res 2017 333: 67-73.
17. Monje-Reyna D, García-Hernández LI, Carrillo-Castilla P, Coria-Ávila GA, Toledo-Cárdenas MR, Hernández-Aguilar ME, Manzo J. Incremento de receptores endocanabinoides cerebelares tras estimulación musical de ratas con autismo inducido posnatalmente. eNeurobiol 2019 23(10): 220219.
18. Crespo CN, Garcia LI, Coria GA, Carrillo P, Hernandez ME, Manzo J. Mejora de las habilidades motoras y cognitivas de niños con autismo después de un periodo prolongado de juego con deportes virtuales. eNeurobiologia 2016 15: 070716.
19. Reynolds S, Millette A, Devine DP. Sensory and motor characterization in the postnatal valproate rat model of autism. Dev Neurosci-basel 2012 34(2-3): 258-267.
20. Cohen BI. Use of a GABA-transaminase agonist for treatment of infantile autism. Med Hypotheses 2002 59(1): 115-116.
21. Oblak AL, Gibbs TT, Blatt GJ. Reduced GABAA receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Res 2011 1380: 218-228.
22. Behuet S, Cremer JN, Cremer M, Palomero-Gallagher N, Zilles K, Amunts K. Developmental changes of glutamate and GABA receptor densities in Wistar rats. Front Neuroanat 2019 13: 100.
23. Barnes EM. Use-dependent regulation of GABAa receptors. Int Rev Neurobiol 1996 39:53-76.
24. Perez-Pouchoulen M, Miquel M, Saft P, Brug B, Toledo R, Hernandez ME, Manzo J. Prenatal exposure to sodium valproate alters androgen receptor expression in the developing cerebellum in a region and age specific manner in male and female rats. Int J Dev Neurosci 2016 53: 46-52.
25. Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. Why are autism spectrum conditions more prevalent in males? Plos Biol 2011 9(6): e1001081.
26. Wang M. Neurosteroids and GABA-A receptor function. Front Endocrinol 2011 2: 44.
27. Fernández-Guasti A, Martínez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrino 2005 30(8): 762-770.
28. Xu Q, Yang JW, Cao Y, Zhang LW, Zeng XH, Li F, Du SQ, Wang LP, Liu CZ. Acupuncture improves locomotor function by enhancing GABA receptor expression in transient focal cerebral ischemia rats. Neurosci Lett 2015 588: 88-94.
29. Yoon SS, Kim H, Choi KH, Lee BH, Lee YK, Lim SC, Choi SH, Hwang M, Kim KJ, Yang CH. Acupuncture suppresses morphine self-administration through the GABA receptors. Brain Res Bull 2010 81(6): 625-630.
30. Ghit A, Assal D, Al-Shami AS, Hussein DEE. GABAa receptors: structure, function, pharmacology, and related disorders. J Genetic Eng Biotechnology 2021 19(1): 123.
31. Evenseth LSM, Gabrielsen M, Sylte I. The GABAb receptor—structure, ligand binding and drug development. Molecules 2020 25(13): 3093.
32. Fritschy J, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAa-receptor subtypes during postnatal development: An immunohistochemical study. J Neurosci 1994 14(9): 5302-5324.
33. Gaiarsa JL, Porcher C. Emerging neurotrophic role of GABAb receptors in neuronal circuit development. Front Cell Neurosci 2013 7: 206.
34. Pizzarelli R, Cherubini E. Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast 2011 2011: 297153.
| Recibido: 02 de febrero, 2022 | Aceptado: 01 de abril, 2022 |
Instituto de Investigaciones Cerebrales. Universidad Veracruzana. Xalapa, Ver. Mexico. C.P. 91010, Tel: 52 (228) 8 418900 ext. 13067. E-mail: jmanzo@uv.mx
Este es un artículo de libre acceso distribuido bajo los términos de la licencia de Creative Commons, (http://creamasal@unam.mxtivecommons.org/licenses/by-nc/3.0), que permite el uso no comercial, distribución y reproducción en algún medio, siempre que la obra original sea debidamente citada.