La restricción perinatal de alimento altera el aprendizaje de preferencia al alimento en ratas Wistar macho adolescentes
- Inicio
- Comité Editorial
- Lineamientos
- Carta de Cesión de Derechos
- Información Legal
- Acerca de la Revista
- Bases de Datos
- Contacto
- ISSN 2007-3054
- Centro de Investigaciones Cerebrales
Universidad Veracruzana
Fernández Regina, Regalado Mirelta, Torrero Carmen, Rubio Lorena, Salas Manuel
Department of Developmental Neurobiology and Neurophysiology, Institute of Neurobiology, Universidad Nacional Autónoma de México, Campus UNAM Juriquilla, Querétaro, México.
Resumen/Abstract
Introducción
Material y métodos
Resultados
Discusión
Conclusiones
Agradecimientos
Referencias
Correspondencia
El efecto de la desnutrición temprana sobre el aprendizaje social de preferencia por el alimento se analizó en ratas adolescentes. En los días 1 y 2 se evaluaron las visitas al alimento y la ingesta de cocoa o canela en una prueba de preferencia al alimento por un demostrador (DEM) control (CG) o desnutrido (UG). En el día 3 en una caja adyacente al DEM se registró, el número de miradas y el autoaseo que realiza el OBS. Durante el encuentro social del DEM+OBS, se evaluó el número de contactos con la cabeza dirigidos al DEM, olfateos y autoaseo del OBS. En el día 4, se registraron las visitas al alimento y la ingesta de cocoa o canela por el OBS CG y UG en la prueba de preferencia al alimento. Previo al encuentro social el OBS desnutrido redujo significativamente las miradas al DEM, y ambos DEM CG y UG prefirieron la canela. Durante el encuentro social en el OBS UG hubo un incremento significativo en el autoaseo y contactos con la cabeza, con menos olfateos que el UG. En el día 4 el OBS UG visitó más el alimento con sabor a cocoa y sólo el OBS UG prefirió la canela que el CG. Los resultados sugieren que la desnutrición temprana altera la habilidad para transferir a un compañero la preferencia por el alimento.
Palabras clave: Preferencia al alimento, Rata, Aprendizaje social, Desnutrición.
The effects of early undernutrition on the social learning of food preference in adolescent rats were analyzed. On days 1, 2 of a food-preference test the visits to food and the cocoa vs. cinnamon ingestion by demonstrator (DEM) control (CG) or an underfed (UG) were measured. On day 3 in an adjacent cage to the DEM, the number of sights and self-grooming by an observer (OBS) was also noted. Thereafter, during a DEM + OBS social encounter, the number of head contacts, snout sniffing, and self-grooming by an OBS were measured. On day 4 of a food-preference test the visits to food and the cocoa vs. cinnamon ingestion by an OBS CG or UG were scored. Before the social encounter the OBS underfed significantly reduced the sights to the DEM, and both DEM CG and UG rats preferred the cinnamon. During the social encounter there were significant increases in the OBS UG in self-grooming and head contacts, with less snout sniffing than CG. On day 4 the OBS UG visited more the cocoa food and only the OBS UG preferred more cinnamon than controls. The findings suggest that early undernutrition disrupts the ability to transfer food preference to a partner.
Key words: Food Preference, Rat, Social Learning, Undernutrition.
In altricial species, the perinatal period which includes gestation and lactation is crucial to program the development and maturation of many tissues. In mammals early food restriction results in anatomical and functional brain alterations that impair short- and long-term behavioral, biochemical and electrophysiological coding signals affecting the expression of cognitive processes such as emotion, memory, selective attentiveness, visuo-spatial learning, and maternal behavior, among others.1-5 These brain alterations associated with perinatal undernutrition include at least, reduction of the density of dendrites and spines, the number of neurons and synaptic contacts in various forebrain brain areas involve in complex cognitive processes and brain disorders.4,6-9 Early food restriction may also interfere with the development of neuronal gustatory relays, as evidenced by delayed expression of papillary receptors, brain weight reductions, dendritic arbor prolongations and neuronal soma hypoplasia at the solitary tract nucleus, parabrachial nuclei, the amygdala, and the insular cortex, which may interfere with the neuronal afferent electrical coding integration of the gustatory and olfactory signals at the long-term memory of gustative experience.10-16
In altricial species the gustatory experience begins during the prenatal period when the fetus can shape food preferences because the cues are transmitted from the maternal dietary components to the amniotic fluid and then to the fetus.12,17,18 The newborn rats are able to discriminate tastes and generate gustofacial responses because the neuronal circuits are already operating in order to gain associative gustatory experience and utilize the preference-aversion processes for feeding behaviors.19,20 Moreover, a deficiency in some components of the normal diet rat alters the preference for taste solutions.21,22 On the other hand, early undernutrition delays the development of social behavioral interaction, with reduced body contacts and social cue exploration and no social self-grooming or aggressive behaviors.23 In mammals, the behavior of individuals provides a rich source of information that other conspecifics can use to improve their behavior without direct experience, and this process is referred to as social learning.24,25 The social transmission of food preferences task is a natural test of paired associative learning ability that takes advantage of the innate ability of a rat to employ olfactory cues to avoid poisoned food. In this task the observer rat interacts with the demonstrator subject who has consumed a flavored rat chow that the observer then prefers as the target food when given a choice between the normal and flavored chow.24 Because perinatal food restriction interferes with social interaction, the development of gustatory papillae and neuronal relays, and the synaptic plasticity needed for the pups to obtain the early sensory experience, the current study analyses if perinatally underfed male rats showed deficiencies during adolescence in social learning of food preferences from a demonstrator DEM to an observer OBS subject. This process is fundamental for social development and for the hedonic impact of integrating gustatory information throughout the sensory limbic relays that contribute to food selection.
2.1. Experimental animals
Subjects were adolescent male Wistar rats (115-120 g), descendants of a stock originally obtained from Harlan Sprague-Dawley (IN, USA). The animals were maintained in an automatically controlled room at 22 ± 2º C, 50% humidity on a 12-h/12-h light/dark cycle (lights on at 07:00), with water and food (5001 rodent Purina chow) ad libitum. For mating, two males were placed in standard translucent plastic cages (60 x 50 x 20 cm3) containing four virgin females (200-250 g). One week before parturition sperm-positive females were placed individually in plastic maternity cages (50 x 30 x 20 cm3) with grill tops and wood shavings as nesting material. The day after birth is referred to as PD 0, and 24 h later pups from different litters were weighed and sexed four females and four males from each litter were randomly distributed among dams in order to minimize genetic and nutritional differences that might influence the experimental results. The presence of the bilateral thoracic and abdominal line of nipples and the shorter anogenital distance in the females were used as criteria for sex recognition.26 Animal care and protocols were approved by local Animal Committees and adhered to the National Research Council Guidelines for the care and use of mammals, and also by the Norma Oficial Mexicana (NOM-062-ZOO-1999) of México.27,28
2.2. Nutritional procedures
2.2.1. The control group (CG)
The CG consisted of 6 adolescent male rats obtained from 4 normally fed litters, nourished by well-fed dams with free access to food (5001 rodent Purina chow) and water. After birth, CG rats were fed and handled by interchanging a pair of normally lactating mothers (one of them with a sham nipple ligature) every 12 h (at 08:00 and 20:00 h) between the litters as described elsewhere.13 This experimental paradigm permits adequate nursing of pups and care from the mother (body licking, retrieving, anogenital stimulation, physical contacts, crouching position over the pups, etc.). To evaluate the effects of the nutritional paradigm on physical growth, body weights of animals with different experimental treatments and ages were noted.
2.2.2. The undernourished group (UG)
This group contained 6 adolescent male rats obtained from at least 4 different litters. The normal chow diet requirement was calculated by measuring the weekly food intake of a group of 6 pregnant control rats (200-250 g) over a 21-day period. The resulting average food intake for each week was the basal level used to calculate the food-intake percentage of the UG females. Thus, mothers were fed from gestational day 6 (G6) to G12 with 50% (7.8 g) of the normal diet (5001 rodent Purina chow), from G13-G18 with 70% (10.9 g), and from G19-G21 with 100% (15.6 g) of the same diet until parturition to avoid resorption of the fetus or cannibalism of pups by the dam. This protocol was chosen because neurogenesis in the brainstem and forebrain structures in the gustatory relays and afferent synaptic connectivity occur primarily from G16 to G21.29 At birth, prenatally undernourished newborns were nursed by two gestationally underfed dams, in one of which the main galactophorous ducts had been tied subcutaneously. To continue the neonatal underfeeding paradigm, these two lactating dams were interchanged every 12 h between litters on postnatal days (PDs) 1-24. This cross-fostering procedure minimizes the effects on the newborns of maternal sensory deprivation that may affect behavioral development. In all cases, four female and four male pups made up a litter for the neonatal feeding. Weaning was performed at PD 25. After this, the pups had free access to solid food (5001 rodent diet) until the flavor-discrimination tests were performed. Animals from both experimental groups were weighed at PDs 1, 5, 10, 15, 20, and 25 to evaluate the effects of dietary treatments on their physical development.
2.3. Behavioral testing
All food-preference tests were performed between 10:00 and 12:00 h in a sound-proof chamber illuminated with red light (60 W), maintained at 23 ± 2º C, and separated from the ambient noise of the main laboratory. The flavored food used to test the food preferences of experimental subjects was prepared as follows: cinnamon-flavored food contained powdered rodent rat chow (495 g) mixed with ground cinnamon (5 g); the cocoa-flavored diet contained powdered rat chow (490 g) mixed with unsweetened powdered cocoa (10 g). The behavioral tests to two flavors were carried out with a minor modification of the procedure previously described for young and adult rats to a single flavor.24 A total of 12 adolescent male subjects was used, including control (n=6) and undernourished (n=6) adolescent male subjects.30 On PDs 33 and 34 experimental rats were exposed to the testing cage over a 20-min span for adaptation. We evaluated if perinatally underfed adolescent male rats between PDs 35 to 38 showed deficiencies in the ability to transfer food-flavor preferences from a DEM rat to an OBS subject. The DEM rat was defined as a single subject given access to two flavored foods in a plastic translucent cage (31 x 23 x 16.5 cm3) after that behavioral experience, the rat had a social encounter (30 min) with an OBS rat allowing transfer of the acquired food experience information to the OBS subject.24On PDs 35, 36 and 37 and prior to food preference testing the DEM control and underfed rats were deprived of food for 23 h to motivate feeding. In all cases, a pair of subjects of the same dietary condition, one DEM and one OBS was exposed to the test.
On the first two days of a food-preference tests (1 h each) the frequency of visits to the food, the amount of food ingested (g), and the frequency of self-grooming by a single DEM control or underfed rat were scored. On the third day a DEM control or underfed rat was tested (1 h) in the presence of an OBS control or underfed rat placed at a distance of 7 cm in a translucent plastic cage (31 x 23 x 16.5 cm3), and the DEM rat´s frequency of visits and food consumption were noted. Moreover, the frequencies of the OBS sights directed to the DEM and of OBS self-grooming were also measured.
In order to permit the OBS to obtain direct sensory information (gustatory, visual, auditory, olfactory, tactile, etc.) from the DEM on the same third day of testing, there was a 30-min social encounter between a DEM and an OBS (DEM + OBS), either control or undernourished subjects, with each in a translucent plastic cage. Moreover, during this social encounter the frequencies of head contacts and sniffing of the snout elicited by the DEM and the frequency of self-grooming observed in the OBS were also evaluated. These measurements reflect the social interaction between pairs of rats, as previously described.24 Finally, on the fourth day of social learning of food preferences testing the behavioral responses including the frequency of visits, food consumption, and self-grooming of an OBS underfed or control were evaluated for 1 h (Figure 1). Because the self-grooming performance is a measure of level of the novelty and emotion in underfed rats that may be influenced by different environmental contexts, the expression of this behavioral parameter was evaluated along the different days of testing.31
2.4. Statistical analysis
Experimental measurements were compared with the Statistical Package version 6. To compare score differences between ages, dietary treatments and flavored-food exposures, the following separate statistical analyses were used: a) body weight scores of pups from birth to weaning were compared in a two-way ANOVA, 2(nutritional regimes) with repeated measures in one factor x 6(ages); b) the DEM rats´ frequency of visits on days 1, 2, and 3 of testing were compared with a three-way ANOVA, 2(nutritional treatments) x 2(flavored foods) x 3(days of testing); additionally in the OBS rats, score differences were compared with a two-way ANOVA, 2(nutritional regimes) x 2(flavored foods); c) food consumption on days 1 and 3 of the DEM and on day 4 of the OBS, were compared with a two-way ANOVA, 2(nutritional regimes) x 2(flavored foods); d) frequency of sights, self-grooming, head contacts, and social sniffing by the OBS subjects before and during the social encounter were analyzed with a one-way ANOVA 2(nutritional treatments). Statistical comparisons between groups over time were made using the Fisher LSD post hoc test. The threshold for significance was set at p < 0.05.
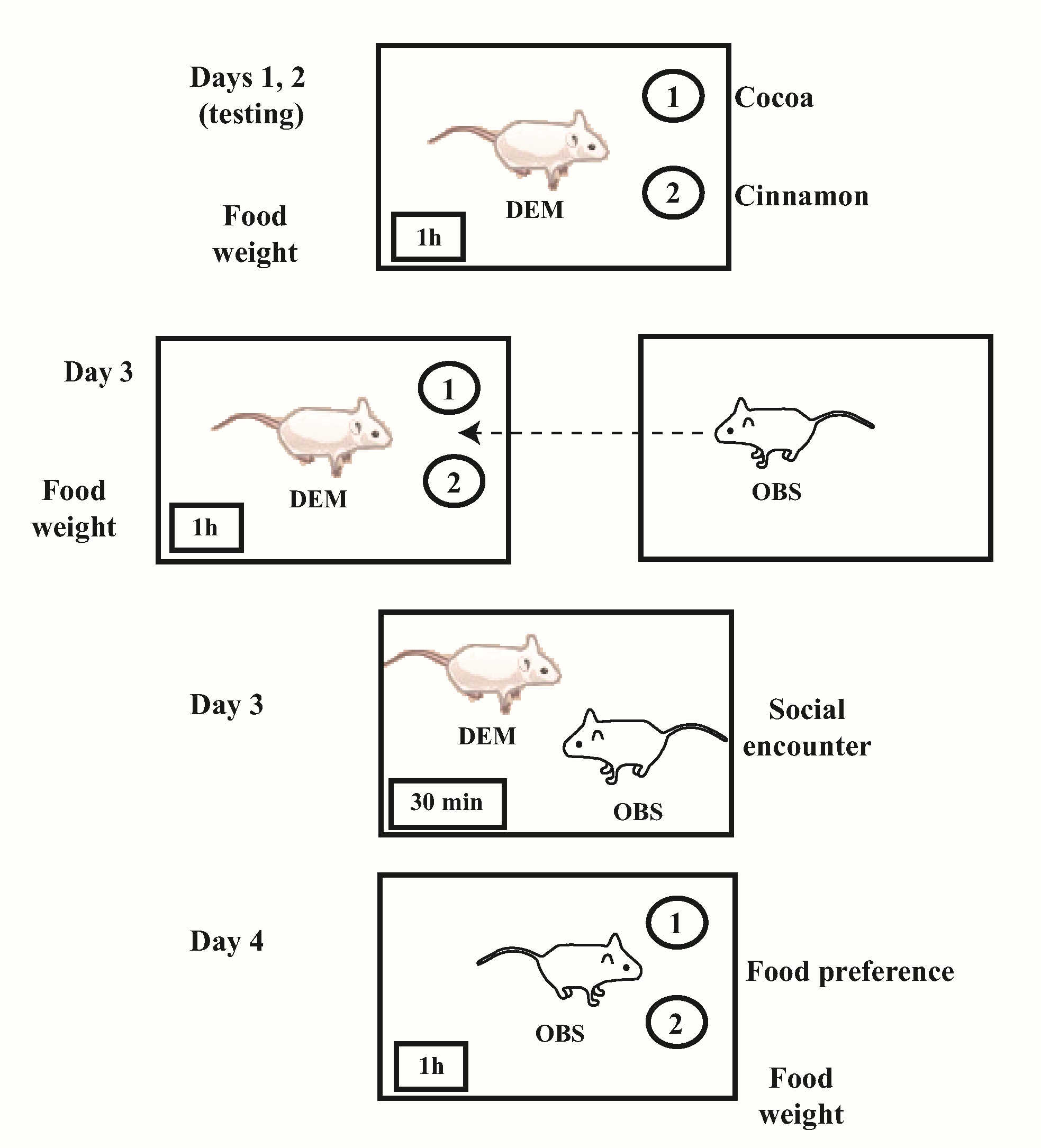
Figure 1. Scheme of the experimental paradigm used. DEM and OBS indicate subjects involved in the test; circled numbers indicate, 1) cocoa-flavored food; 2) cinnamon-flavored food. Interrupted arrow indicates the direction of searching information by the OBS.
3.1. Body weight effects
The ANOVA comparisons indicated significant body weight reductions of rats associated with diet, F (1, 35) = 37.55, p < 0.0001; and with age, F (5, 175) = 638.69, p < 0.0001, and a significant interaction diet x age, F (5, 175) = 8.39, p < 0.0001. Post hoc comparisons at each developmental age showed a significantly (p < 0.05) lower body weight in the UG rats at PDs 10, 15, 20, and 25 compared to the CG subjects (Table 1). The data indicated that the undernourishment paradigm consistently interfered with the physical growth of the UG animals during this period of life.
3.2. Behavioral testing effects
The frequencies of visits to the cocoa and cinnamon flavors in the DEM underfed vs. control subjects were not significantly different. However, there was a significant reduction over time with higher frequency on day 1 and with a progressive decline on test days 2, and 3, F(2, 20) = 14.20, p < 0.001, (Fig. 2A). It seems clear that a progressive loss of interest is expressed in both DEM control and undernourished animals. The cumulative effects (Total) of undernutrition significantly reduced the average number of visits only to the cinnamon-flavored food, F (1, 10) = 7.55, p < 0.020, (Figure 2B). The DEM groups UG and CG did not differ in their consumption of the flavored foods, F (1, 10) = 2.947, p = 0.116, (Figure 2C) or their frequency of self-grooming, F (1, 10) = 0.09, p = 0.760.
Table 1. Mean values ± SEM of body weight (g) in CG and UG rats (n= 6/group) during development.
| Age (days) |
Groups |
|
|
|---|---|---|---|
| CG |
UG |
p < |
|
1 |
6.67 ± 0.11 |
5.83 ± 0.10 |
NS |
5 |
10.59 ± 0.14 |
8.05 ± 0.28 |
NS |
10 |
20.36 ± 1.17 |
14.11 ± 0.39 |
* |
15 |
25.15 ± 1.34 |
20.00 ± 0.72 |
* |
20 |
36.66 ± 1.61 |
25.75 ± 1.13 |
* |
25 |
53.15±2.39 |
45.28±1.23 |
* |
| Factor |
df |
F |
p < |
| (A) Diet |
1, 35 |
37.55 |
0.0001 |
| (B) Age |
5, 175 |
638.69 |
0.0001 |
| A x B |
5, 175 |
8.39 |
0.0001 |
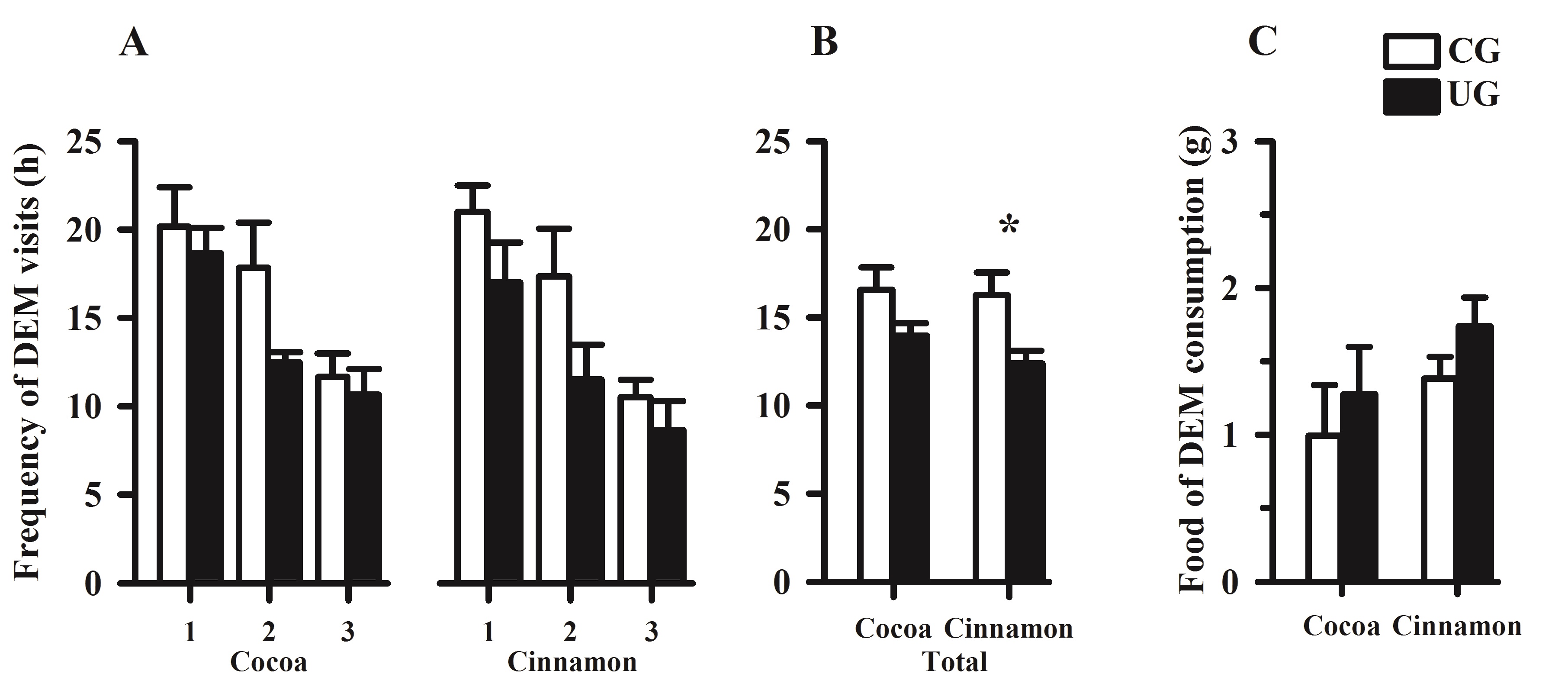
Figure 2. (A) Mean frequency ± SEM of visits to the food (cocoa and cinnamon) during the initial 3 days of food-preference tests in the DEM subjects (CG and UG). (B) Mean cumulative effects of undernutrition along the days of testing. *Significant difference between DEM groups CG and UG in visits to cinnamon, p < 0.05; (C) Mean ± SEM on day 1 of food consumption by DEM groups CG and UG.
On the third day of testing and before the social encounter, the one-way ANOVA showed a decreased frequency of sights of the OBS underfed directed to a DEM control subject, F(1, 10) = 5.66, p < 0.038, (Figure 3A). Additionally, the self-grooming frequency increased significantly in the OBS underfed rats compared with the OBS control subjects, F(1, 10) = 7.36, p < 0.021, (Figure 3B). During the social encounter there were significantly more head contacts by the OBS underfed directed to the head of the DEM partner, F(1, 10) = 5.03, p < 0.048, (Figure 3C) and the frequency of the snout sniffing by of the OBS underfed directed to the snout of the DEM was significantly reduced, F(1, 10) = 19.56, p < 0.001, (Figure 3D), but there were no significant differences between the OBS UG and CG animals in the frequency of self-grooming bouts, F(1, 10) = 0.62, p= 0.447. After the social encounter on day 4 of testing, the frequency of visits between the OBS control and underfeed rats indicated, a significant increase in the preference for the cocoa-flavored food by the UG rats, F (1, 10) = 12.53, p < 0.005, (Figure 3E), with no differences between groups in the cinnamon food preference, and there was a significant interaction between flavor x diet factors, F (1, 10) = 23.72, p < 0.006.
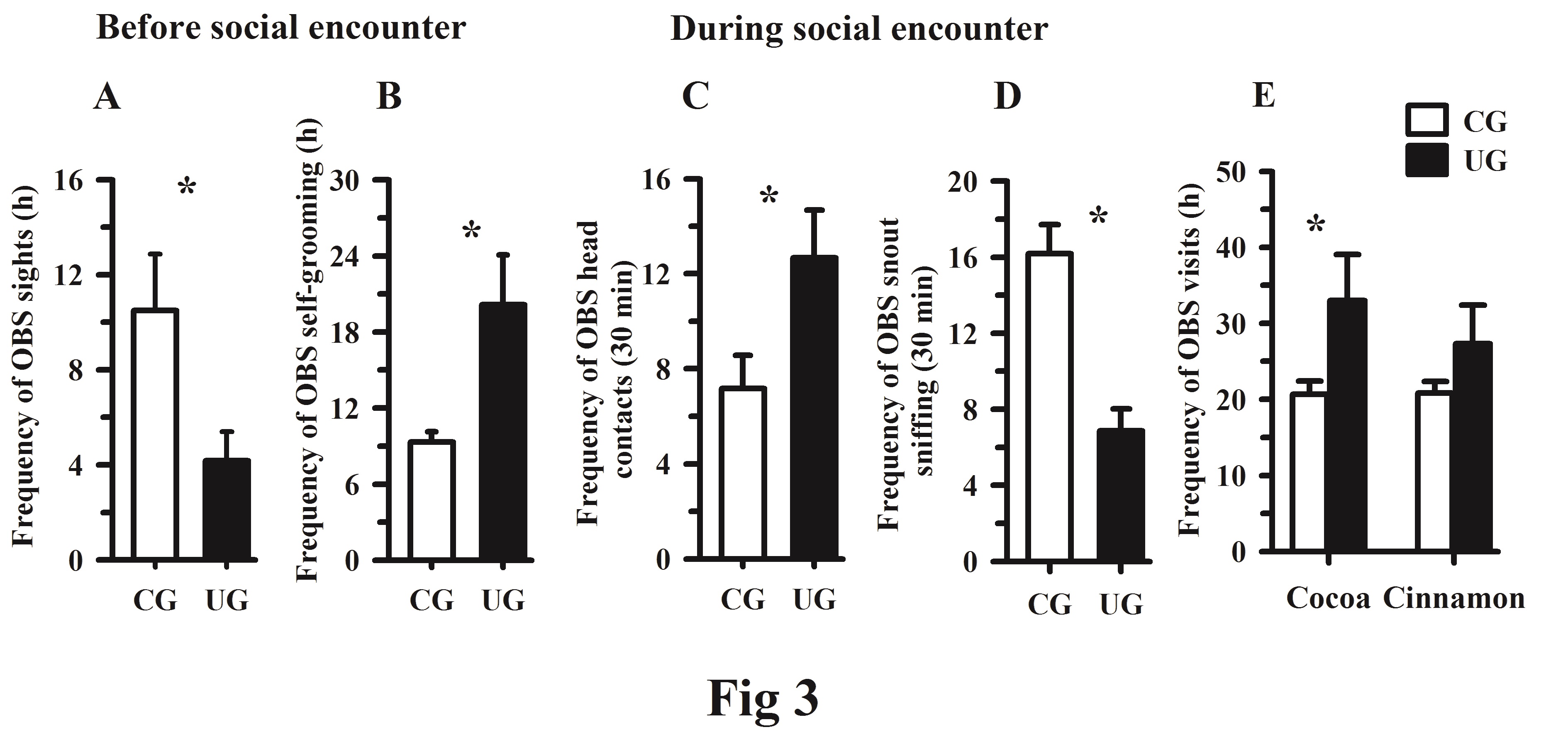
Figure 3.Before social encounter: (A) Mean frequency values of ± SEM of sights by an OBS control or underfed directed to one DEM subject (CG or UG); (B) Mean frequency of self-grooming bouts in OBS subjects, CG vs. UG rats. During social encounter: (C) Mean frequency values ± SEM of head contacts; and (D) Snout sniffing values between the animals, OBS (CG vs. UG). After the social encounter: (E) Frequency of visits by OBS subjects (CG and UG) to the flavored food. *Significant differences associated with the dietary group, p < 0.05.
On day 3 of testing consumption of the two-flavored foods did not differ significantly between the DEM control and underfed rats. However, when the CG and UG cinnamon food consumption vs. with the CG and UG cocoa food consumption, consumption of cinnamon-flavored food was significantly greater, F(1, 10) = 5.02, p < 0.048, with no interaction between factors, (Figure 4A). Furthermore, in the UG rats on day 4 of the test cocoa-flavored food consumption decreased but cinnamon-flavored food consumption increased significantly, F (1, 10) = 12.53, p < 0.005, and a significant interaction between the flavored food x diet, F (1, 10) = 23.72, p < 0.006 was observed, (Figure 4B). The frequencies of self-grooming bouts did not differ significantly between the CG and UG rats, F (1, 10) = 0.12, p = 0.727.
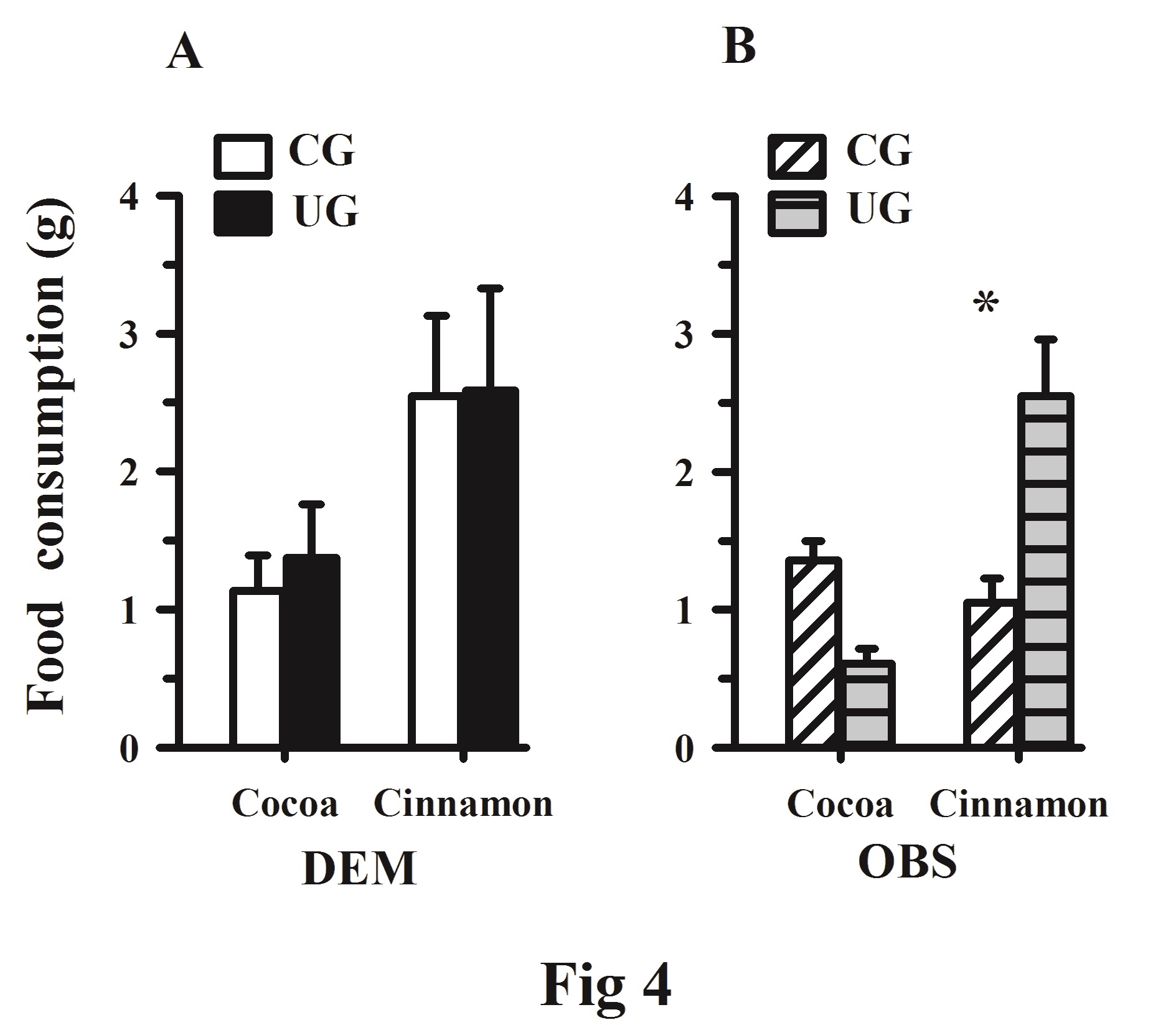
Figure 4. (A) Mean ± SEM food consumption (cocoa and cinnamon) on day 3 of testing in the DEM subjects (CG vs. UG). (B) Mean ± SEM food-consumption on day 4 of testing in OBS groups (CG vs. UG). *Significant differences between dietary groups, p < 0.05. Note the increased cinnamon consumption in the OBS UG rats.
The present findings indicate that pre- and neonatal undernutrition interferes with the physical development of the UG animals including their body weight. These alterations are consistent with earlier reports, suggesting that a number of factors, such as the abnormal structure and function of the placenta and the altered mother-litter interactions, reduced the physical contacts and the licking of the pups. Moreover, undernutrition, disrupts the release of various hormones and growth factors that promote body and brain synaptic development, which constitutes evidence of the noxious effects of perinatal food restriction.32-35
The frequency of visits to the cocoa or cinnamon flavors in the DEM underfed vs. the DEM control subjects along the days of testing was not significantly different. However, these frequencies were high on day 1, and they progressively declined on the second and third days of testing, suggesting a reduced attentiveness to sensory cues, including food, in both the DEM control and undernourished animals; the cumulative effects of undernutrition with increasing age (Total) caused a significant reduction in visits (p < 0.05) only to the cinnamon-flavored food. These findings showed that the attentive brain processes taking place in the telencephalic structures while visiting the food were present in both the DEM control and underfed rats, with significant decreases in the UG subjects in response to the cinnamon flavor. These effects are in line with studies suggesting that early undernutrition interferes with brain organization and causes abnormal synaptic plasticity and function of brain areas related to attentiveness, visuo-spatial learning, exploratory activity, and social cognitive responsiveness that may affect food preferences.5,10,35-38
The data obtained on the third day of tests showed that the number of sights of OBS control subjects directed to the DEM rat was significantly increased, while the result was opposite for interactions of an OBS underfed with a DEM animal. Furthermore, in the OBS UG group the frequency of self-grooming bouts was consistently increased, possibly because of the novel cage environment during the test. These results are consistent with previous studies showing that early undernutrition results in poor environmental exploration and increased self-grooming activity, which may be due to impaired development of cognitive networks; this may include a reduction in the number of granule cells that inhibit cortico-subcortical brain mechanisms taking place early in life as previously described.3639-42
Another point of interest concerns the behavioral interactions between the DEM and OBS subjects during the social learning between the subjects. The data showed that in OBS the UG rats significantly increased the head contacts, but they reduced the frequency of the snout sniffing directed to the DEM animals when searching for olfactory and gustatory food fragments, thereby reducing the opportunity to transfer sensory information to the DEM partner;24 however, the current findings could be related to the effects of early food restriction as a relevant stressor that interferes with the synaptic organization of cognitive brain areas and the increased glucocorticoid release that reprograms the HPA axis of the newborn resulting, at later ages, in non-adaptive social behaviors.43,44
Measurements of food consumption used to determine preference for cocoa or cinnamon in DEM control and underfed rats did not indicate significant differences. However, on day 3 of testing, both the CG and UG experimental groups showed a non-significant increase preference for the cinnamon flavor between them (Figure 4A). The effects of OBS undernutrition on the underfed significantly increased their preference for the cinnamon flavor with no significant effect for the control subjects (Figure 4B). Several anatomical studies using this underfeeding paradigm or specific brain lesions have shown significantly reduced dendritic arborizations and spine density, with a significant decrease in the perimeter and cross-sectional area of the neuronal perikarya at different levels of the gustatory pathway, that may disrupt the activation of different neurons related to the spatiotemporal patterns of cinnamon stimulation, resulting in altered cognitive processes.14,45 In type two diabetes patients, the cinnamon sweet preference threshold as in UG subjects may be increased, because the subcortical ascending information is reduced and therefore less able to activate a cortical inhibitory mechanism that modulates the preference for sweet food.15 These findings could be also related to the long-term influence of perinatal undernutrition that results in motor hyperactivity and in delayed maturation of the olfactory glomeruli, the mitral cell, and the hippocampal dentate gyrus in the UG rats; these deficits interfere with the social learning of food preferences to the partner of both the olfactory and gustatory preference.46,47 However, further studies are needed to investigate the extent to which the anatomical changes associated with early undernutrition are reversible, and to study the possible functional influence of the synaptic alterations at different sensory relays underlying food-preference ability. These findings suggest that early undernutrition disrupts the rat´s ability to social learning of food preference to a partner during the adolescent stage.
The present data indicate that before the social encounter the DEM control visits the cinnamon flavor more often than the DEM underfed without preference for both flavors. During the encounter on test day 3 the OBS underfed give more head contacts explored and sniffed the snout of the partner less often. However, on day 3 of testing, both the CG and UG experimental groups showed a non-significant increase preference for the cinnamon flavor between them. The effects of undernutrition on the OBS underfed on day 4 significantly increased their preference for the cinnamon flavor with no significant effect for the CG subjects. The findings suggest that early undernutrition disrupts the rat´s ability to transfer the flavor preference to a partner during the adolescent stage.
The work was partly supported by DGAPA/UNAM, IN200413, and CONACyT No. 284452 scholarship to R F. V-M. We thank Dr. Dorothy Pless for editorial assistance and suggestions, and Dr. B. Rangel for helpful comments and discussions.
7. Conflict of interest
Authors of the present manuscript declare no conflict of interest.
- Bousalham R, Benazzouz B, Hessni A El, Ouichou A, Mesfioui A. Maternal separation affects mothers ’ affective and reproductive behaviors as well as second offspring’s. Emotionality Test 2013 3: 409–414.
- Brock JW and Prasad C. Alterations in dendritic spine density in the rat brain associated with protein malnutrition. Dev Brain Res 1992 66: 266–269.
- Campbell LF and Bedi KS. The effects of undernutrition during early life on spatial learning. Physiol Behav 1989 45: 883–890.
- Pascual R and Zamora-Leon SP. Effects of neonatal maternal deprivation and postweaning environmental complexity on dendritic morphology of prefrontal pyramidal neurons in the rat. Acta Neurobiol Exp (Wars) 2007 67: 471–479.
- Sullivan RM and Brake WG. What the rodent prefrontal cortex can teach us about attention-deficit/ hyperactivity disorder: The critical role of early developmental events on prefrontal function. Behav Brain Res 2003 146: 43-55.
- Bunney WE and Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Rev 2000 31: 138–146.
- Coupé B, Dutriez-Casteloot I, Breton C, Lefèvre F, Mairesse J, Dickes-Coopman A, Silhol M, Tapia-Arancibia L, Lesage J, Vieau D. Perinatal undernutrition modifies cell proliferation and brain-derived neurotrophic factor levels during critical time-windows for hypothalamic and hippocampal development in the male rat. J Neuroendocrinol 2009 21: 40–48.
- Florian ML and Nunes ML. Effects of intra-uterine and early extra-uterine malnutrition on seizure threshold and hippocampal morphometry of pup rats. Nutr Neurosci 2010 13: 265–273.
- Torrero C, Regalado M, Rubio L, Salas M. Effects of neonatal undernutrition on development of the dorsolateral prefrontal cortex pyramidal Cells in the Rat. J Behav Brain Sci 2014 4: 49–57.
- Hill DL and Almli CR. Parabrachial nuclei damage in infant rats produces residual deficits in gustatory preferences/aversions and sodium appetite. Dev Psychobiol 1983 16: 519–533.
- Kinzeler NR and Travers SP. Licking and gaping elicited by microstimulation of the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 2008 295: R436–R448.
- Portella AK, Kajantie E, Hovi P, Desai M, Ross MG, Goldani MZ, Roseboom TJ, Silveira PP. Effects of in utero conditions on adult feeding preferences. J Dev Orig Health Dis 2012 3: 140-152.
- Rubio L, Torrero C, Regalado M, Salas M. Alterations in the solitary tract nucleus of the rat following perinatal food restriction and subsequent nutritional rehabilitation. Nutr Neurosci 2004 7: 291–300.
- Sakai N and Imada S. Bilateral lesions of the insular cortex or of the prefrontal cortex block the association between taste and odor in the rat. Neurobiol Learn Mem 2003 80: 24–31.
- Salas M, Torrero C, Rubio L, Regalado M. Effects of perinatal undernutrition on the development of neurons in the rat insular cortex. Nutr Neurosci 2012 15: 20-25.
- Torrero C, Regalado M, Rubio L, Salas M. Parabrachial neuron development : Effects of pre- and neonatal undernutrition in the rat. Open J Mol Intgr Physiol 2012 2: 112–118.
- Mennella JA. Ontogeny of taste preferences: Basic biology and implications for health1-5. Am J Clin Nutr 2014 99: 704S-711S.
- Stickrod G, Kimble DP, Smotherman WP. In utero taste/odor aversion conditioning in the rat. Physiol Behav 1982 28: 5–7.
- Harada S, Yamaguchi K, Kanemaru N, Kasahara Y. Maturation of taste buds on the soft palate of the postnatal rat. Physiol Behav 2000 68: 333–339.
- Steiner JE. The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept 1973 4: 254–278.
- Chan MM and Kare MR. Effect of vitamin B-6 deficiency on preference for several taste solutions in the rat. J Nutr 1979 109: 339–344.
- Reifen R, Agami O, Weiser H, Biesalski H, Naim M. Impaired responses to sweet taste in vitamin A-deficient rats. Metabolism 1998 47: 1–2.
- Fraňková S. Effect of protein-calorie malnutrition on the development of social behavior in rats. Dev Psychobiol 1973 6: 33–43.
- Galef BG. Social learning of food preferences in rodents: rapid appetitive learning. Curr Protoc Neurosci 2003 Chapter 8, Unit 8.5D.
- Gariépy J-F, Watson KK, Du E, Xie DL, Erb J, Amasino D, Platt ML. Social learning in humans and other animals. Front Neurosci 2014 8: 1-13.
- Vandenbergh JG. Prenatal hormone exposure and sexual variation. Am Sci 2003 91: 218–225.
- National Research Council. Guidelines for the Care and Use of Mammals En: National Research Council of the National Academies, Editor. Neuroscience and Behavioral Research. Washington DC: National Acadmeies Press. 2003 Pp 209.
- Norma Oficial Mexicana (NOM-062-ZOO-1999).
- Altman J and Bayer SA. Atlas of prenatal rat brain development. 1995.
- Sengupta P. The laboratory rat: Relating its age with human’s. Int J Prev Med 2013 4: 624-630.
- Massaro TF, Levitsky DA, Barnes RH. Early protein malnutrition in the rat: behavioral changes during rehabilitation. Dev Psychobiol 1977 10: 105–111.
- Álaez C, Calvo R, Obregon MJ, Pascual-Leone AM. Thyroid hormones and 5’-deiodinase activity in neonatal undernourished rats. Endocrinology 1992 130: 773–779.
- Evoniuk GE, Kuhn CM, Schanberg SM. The effect of tactile stimulation on serum growth hormone and tissue ornithine decarboxylase activity during maternal deprivation in rat pups. Commun. Psychopharmacol 1979 3: 363–370.
- Kuhn CM, Butler SR, Schanberg SM. Selective depression of serum growth hormone during maternal deprivation in rat pups. Science 1978 201: 1034–1036.
- Schanberg SM, Evoniuk G, Kuhn CM. Tactile and nutritional aspects of maternal care: specific regulators of neuroendocrine function and cellular development. Proc Soc Exp Biol Med 1984 175: 135–146.
- Almeida SS, Garcia RA, de Oliveira LM. Effects of early protein malnutrition and repeated testing upon locomotor and exploratory behaviors in the elevated plus-maze. Physiol Behav 1993 54: 749–752.
- Bedi KS. Effects of undernutrition during early life on granule cell numbers in the rat dentate gyrus. J Comp Neurol 1991 311: 425–433.
- Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex 2002 12: 1254–1268.
- Febo M, Felix-Ortiz AC, Johnson TR. Inactivation or inhibition of neuronal activity in the medial prefrontal cortex largely reduces pup retrieval and grouping in maternal rats. Brain Res 2010 1325: 77–88.
- Geva R, Zivan M, Warsha A, Olchik D. Alerting, orienting or executive attention networks: differential patterns of pupil dilations. Front Behav Neurosci 2013 7, 1-11.
- Salas M, Pulido S, Torrero C, Escobar C. Neonatal undernutrition and self-grooming development in the rat: long-term effects. Physiol Behav 1991 50: 567–572.
- Spruijt BM, Cools AR, Gispen WH. The periaqueductal gray: a prerequisite for ACTH-induced excessive grooming. Behav Brain Res 1986 20: 19–25.
- De Kloet ER, Oitzl MS, Joëls M. Stress and cognition: Are corticosteroids good or bad guys? Trends Neurosci 1999 22: 422-426.
- Macrì S and Würbel H. Developmental plasticity of HPA and fear responses in rats: A critical review of the maternal mediation hypothesis. Horm Behav 2006 50: 667-680.
- Froemke RC, Letzkus JJ, Kampa BM, Hang GB, Stuart GJ. Dendritic synapse location and neocortical spike-timing dependent plasticity. Front Synaptic Neurosci 2010 2: 1-14.
- Debassio WA, Kemper TL, Tonkiss J, Galler JR. Effect of prenatal protein deprivation on postnatal granule cell generation in the hippocampal dentate gyrus. Brain Res Bull 1996 41: 379–383.
- Frias C, Torrero C, Regalado M, Salas M. Development of mitral cells and olfactory bulb layers in neonatally undernourished rats. Nutr Neurosci 2009 12: 96–104.
| Recibido: 11 de agosto de 2015 | Aceptado: 06 de octubre de 2015 |
Correspondencia: Manuel Salas PhD. Department of Developmental Neurobiology and Neurophysiology, Institute of Neurobiology, Universidad Nacional Autónoma de México, Campus UNAM Juriquilla México, Querétaro México 76230, México. Teléfono: +52-55-5623-4059, Fax: +52-55-5623-4038. E-mail: masal@unam.mx
Este es un artículo de libre acceso distribuido bajo los términos de la licencia de Creative Commons, (http://creativecommons.org/licenses/by-nc/3.0), que permite el uso no comercial, distribución y reproducción en algún medio, siempre que la obra original sea debidamente citada.
